Acute Crit Care.
2022 Feb;37(1):108-117. 10.4266/acc.2021.00332.
Serum lactate levels in cirrhosis and non-cirrhosis patients with septic shock
- Affiliations
-
- 1Division of Critical Care, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- KMID: 2527913
- DOI: http://doi.org/10.4266/acc.2021.00332
Abstract
- Background
In septic shock patients with cirrhosis, impaired liver function might decrease lactate elimination and produce a higher lactate level. This study investigated differences in initial lactate, lactate clearance, and lactate utility between cirrhotic and non-cirrhotic septic shock patients.
Methods
This is a retrospective cohort study conducted at a referral, university-affiliated medical center. We enrolled adults admitted during 2012–2018 who satisfied the septic shock diagnostic criteria of the Surviving Sepsis Campaign: 2012. Patients previously diagnosed with cirrhosis by an imaging modality were classified into the cirrhosis group. The initial lactate levels and levels 6 hours after resuscitation were measured and used to calculate lactate clearance. We compared initial lactate, lactate at 6 hours, and lactate clearance between the cirrhosis and non-cirrhosis groups. The primary outcome was in-hospital mortality.
Results
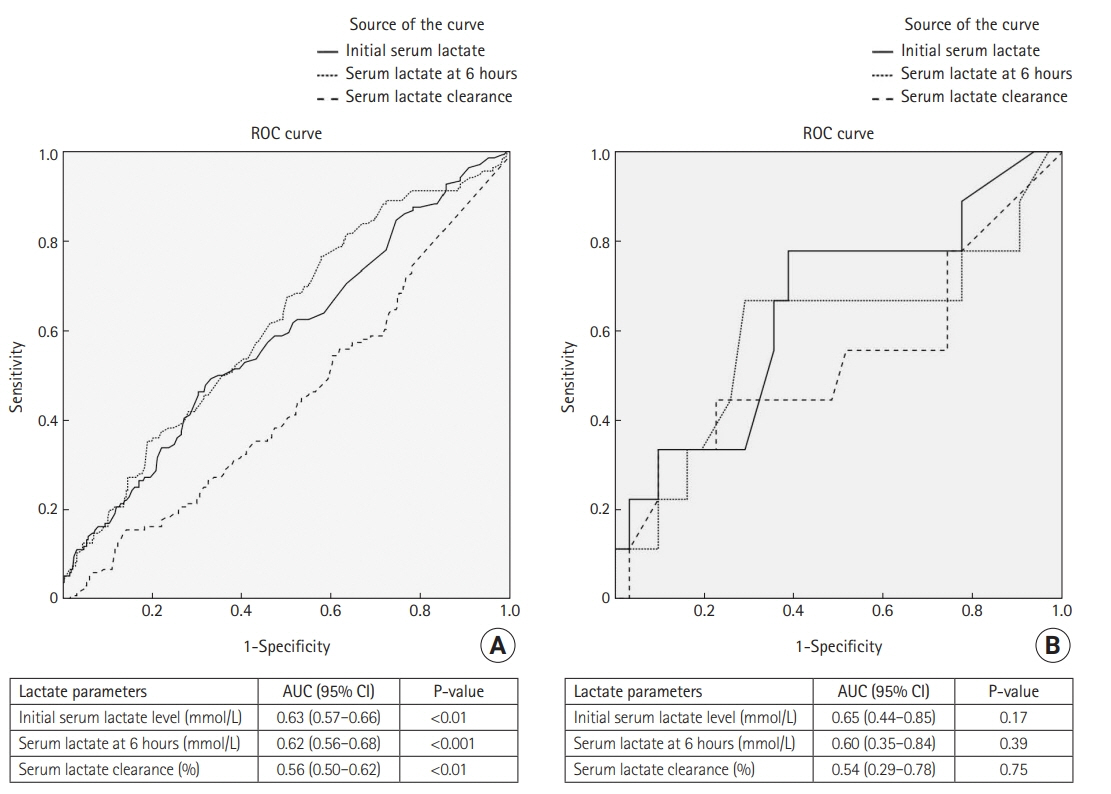
Overall 777 patients were enrolled, of whom 91 had previously been diagnosed with cirrhosis. Initial lactate and lactate at 6 hours were both significantly higher in cirrhosis patients, but there was no difference between the groups in lactate clearance. A receiver operating characteristic curve analysis for predictors of in-hospital mortality revealed cut-off values for initial lactate, lactate at 6 hours, and lactate clearance of >4 mmol/L, >2 mmol/L, and <10%, respectively, among non-cirrhosis patients. Among patients with cirrhosis, the cut-off values predicting in-hospital mortality were >5 mmol/L, >5 mmol/L, and <20%, respectively. Neither lactate level nor lactate clearance was an independent risk factor for in-hospital mortality among cirrhotic and non-cirrhotic septic shock patients.
Conclusions
The initial lactate level and lactate at 6 hours were significantly higher in cirrhosis patients than in non-cirrhosis patients.
Figure
Reference
-
1. Kethireddy S, Bilgili B, Sees A, Kirchner HL, Ofoma UR, Light RB, et al. Culture-negative septic shock compared with culture-positive septic shock: a retrospective cohort study. Crit Care Med. 2018; 46:506–12.2. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:775–87.
Article3. Permpikul C, Tongyoo S, Ratanarat R, Wilachone W, Poompichet A. Impact of septic shock hemodynamic resuscitation guidelines on rapid early volume replacement and reduced mortality. J Med Assoc Thai. 2010; 93 Suppl 1:S102–9.4. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:801–10.
Article5. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA, et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010; 303:739–46.
Article6. Permpikul C, Sringam P, Tongyoo S. Therapeutic goal achievements during severe sepsis and septic shock resuscitation and their association with patients' outcomes. J Med Assoc Thai. 2014; 97 Suppl 3:S176–83.7. Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S. Early use of norepinephrine in septic shock resuscitation (CENSER): a randomized trial. Am J Respir Crit Care Med. 2019; 199:1097–105.
Article8. Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010; 182:752–61.
Article9. Ryoo SM, Lee J, Lee YS, Lee JH, Lim KS, Huh JW, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Crit Care Med. 2018; 46:e489–95.
Article10. Promsin P, Grip J, Norberg Å, Wernerman J, Rooyackers O. Optimal cut-off for hourly lactate reduction in ICU-treated patients with septic shock. Acta Anaesthesiol Scand. 2019; 63:885–94.
Article11. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014; 371:2309–19.
Article12. Fall PJ, Szerlip HM. Lactic acidosis: from sour milk to septic shock. J Intensive Care Med. 2005; 20:255–71.
Article13. Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Curr Opin Crit Care. 2012; 18:267–72.
Article14. Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014; 18:503.
Article15. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637.
Article16. Pattharanitima P, Tongyoo S, Ratanarat R, Wilachone W, Poompichet A, Permpikul C. Correlation of arterial, central venous and capillary lactate levels in septic shock patients. J Med Assoc Thai. 2011; 94 Suppl 1:S175–80.17. Youden WJ. Index for rating diagnostic tests. Cancer. 1950; 3:32–5.
Article18. Jeppesen JB, Mortensen C, Bendtsen F, Møller S. Lactate metabolism in chronic liver disease. Scand J Clin Lab Invest. 2013; 73:293–9.
Article19. Sterling SA, Puskarich MA, Jones AE. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med. 2015; 2:197–202.
Article20. Ha TS, Shin TG, Jo IJ, Hwang SY, Chung CR, Suh GY, et al. Lactate clearance and mortality in septic patients with hepatic dysfunction. Am J Emerg Med. 2016; 34:1011–5.
Article21. Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015; 43:567–73.22. Møller S, Bendtsen F. The pathophysiology of arterial vasodilatation and hyperdynamic circulation in cirrhosis. Liver Int. 2018; 38:570–80.
Article23. Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002; 35:140–8.
Article24. Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993; 18:353–8.
Article25. Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: a critical review and practical guidance. World J Hepatol. 2016; 8:307–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lactate Clearance and Outcome in Septic Shock Patients with Low Level of Initial Lactate
- Recent lactate findings: is repeated serum lactate testing necessary in septic shock patients?
- Serum Lactate Levels in CAPD Patients with Liver Cirrhosis
- Group B Streptococcal Toxic Shock-like Syndrome: A Case Report and Review of the Literature
- Utility of the early lactate area score as a prognostic marker for septic shock patients in the emergency department