Korean J Transplant.
2023 Sep;37(3):170-178. 10.4285/kjt.23.0031.
Factors associated with rituximab-mediated B cell depletion in ABO-incompatible adult living donor liver transplantation
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2546475
- DOI: http://doi.org/10.4285/kjt.23.0031
Abstract
- Background
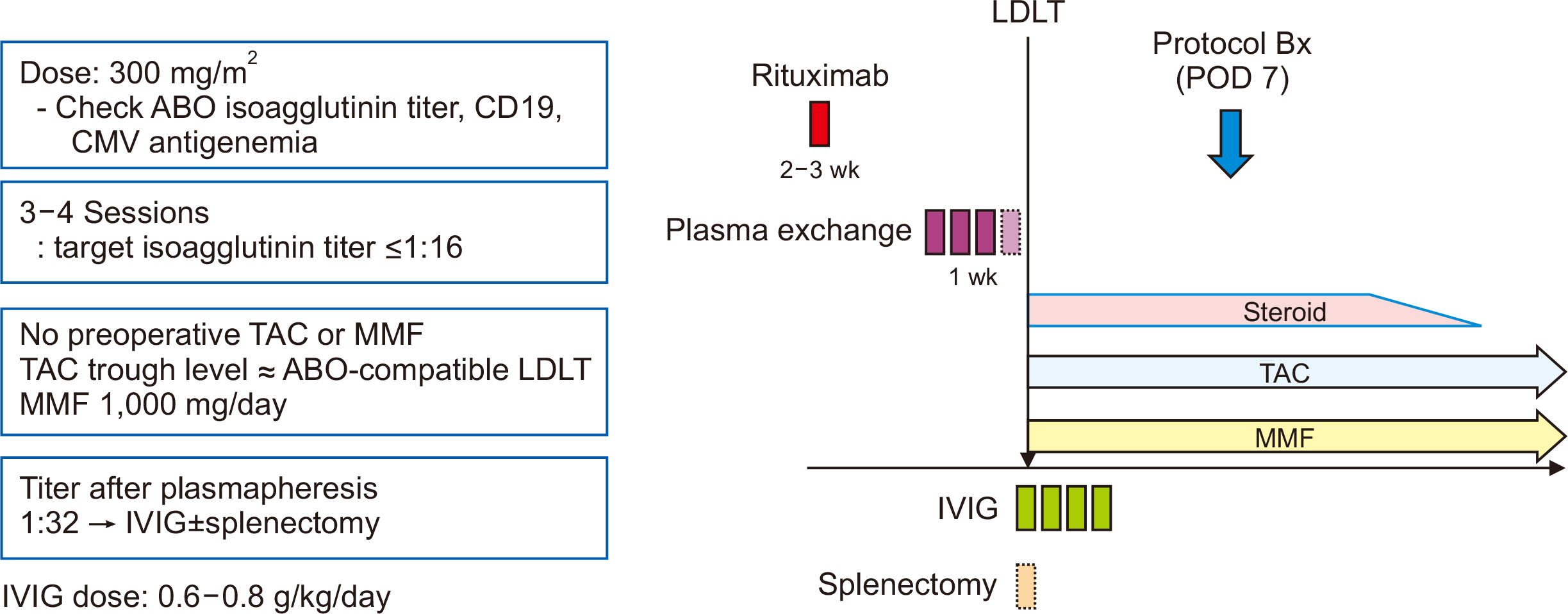
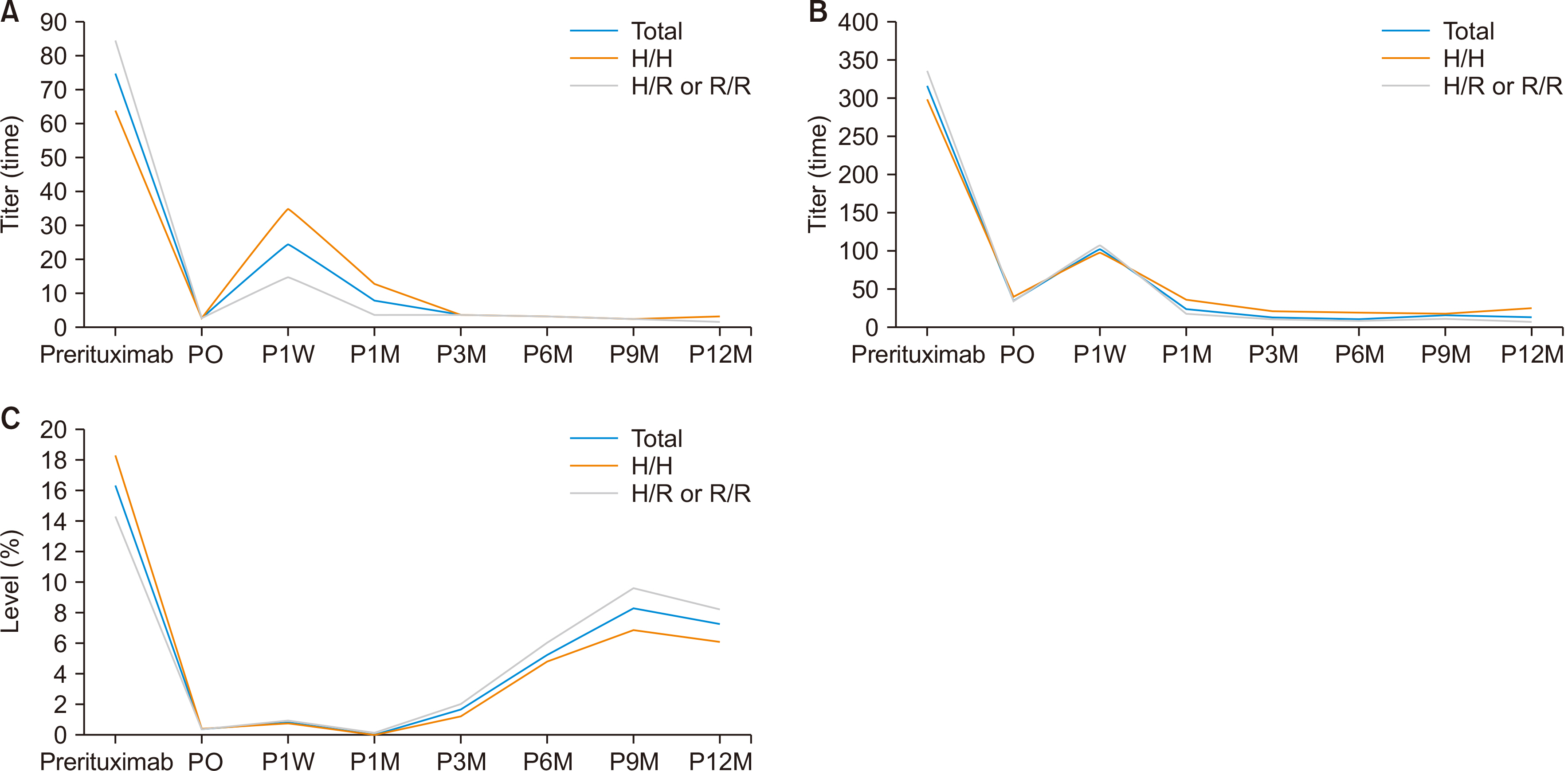
Pretransplant therapies such as rituximab and plasmapheresis have led to an increase in ABO-incompatible (ABOi) living donor liver transplantation (LDLT), thus helping to overcome organ shortages. This study evaluated the changes in anti-A/B titers and CD19 levels over time in patients undergoing ABOi LT and aimed to understand the effect of single-nucleotide polymorphisms (SNPs) in Fc gamma receptor (FcγR) on rituximab therapy.
Methods
Two SNPs of FCGR2A (131H/R) and FCGR3A (158F/V) were identified. The clinical data on 44 patients who underwent ABOi LDLT between May 2019 and October 2021 at Seoul National University Hospital were reviewed retrospectively.
Results
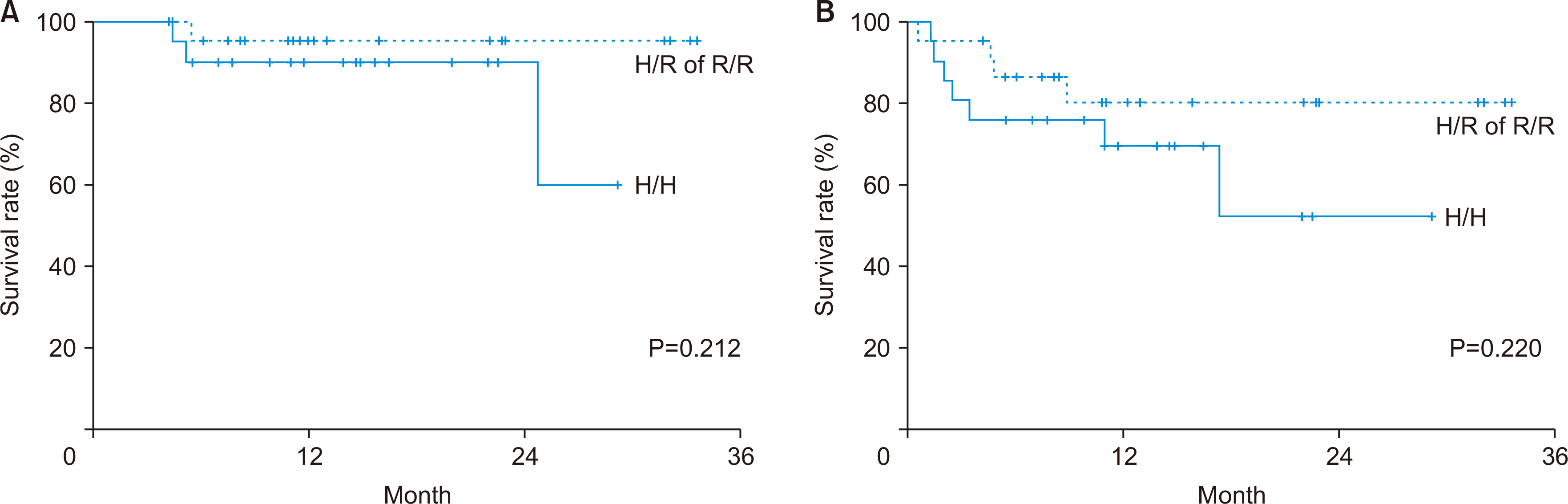
Following desensitization with rituximab and subsequent LDLT, the anti-A/B titer recovered within 1 week, but decreased thereafter. The CD19 level increased at 3 months after LT. The genotyping data for FCGR3A (158F/V) indicated that two patients had the V/V genotype, and 42 had the F/V genotype. In the genotyping data for FCGR2A (131H/R), 21 patients had the H/H genotype, three had the R/R genotype, and 20 had the H/R genotype. However, there were no significant differences in anti-A/B and CD19 levels, bacteremia rates, T cell-mediated rejection, antibody-mediated rejection, or the survival rate among the FCGR2A types.
Conclusions
There were significant changes in the anti-A/B titers and CD19 levels over time in each patient after ABOi LDLT. The difference in outcomes following LT according to the FcγR SNP type for rituximab was unclear. Further studies with larger sample sizes are needed to confirm the effect of FcγR SNPs on rituximab therapy.
Figure
Reference
-
1. Egawa H, Ohdan H, Saito K. 2023; Current status of ABO-incompatible liver transplantation. Transplantation. 107:313–25. DOI: 10.1097/TP.0000000000004250. PMID: 35849558. PMCID: PMC9875847.2. Kok G, Verstegen MM, Houwen RH, Nieuwenhuis EE, Metselaar HJ, Polak WG, et al. 2022; Assessment of human leukocyte antigen matching algorithm PIRCHE-II on liver transplantation outcomes. Liver Transpl. 28:1356–66. DOI: 10.1002/lt.26431. PMID: 35152544. PMCID: PMC9544750.3. Tajima T, Hata K, Kusakabe J, Miyauchi H, Yurugi K, Hishida R, et al. 2022; The impact of human leukocyte antigen mismatch on recipient outcomes in living-donor liver transplantation. Liver Transpl. 28:1588–602. DOI: 10.1002/lt.26511. PMID: 35603526. PMCID: PMC9796617.4. Kim H, Yi NJ, Song EY, Lee K, Lee KW, Lee HW, et al. 2018; Preformed donor-specific antibodies do not affect the 1-year allograft survival in living donor liver transplantation. Clin Transplant. 32:e13244. DOI: 10.1111/ctr.13244. PMID: 29577436.5. Natsuda K, Murokawa T, Lee KW, Yoon KC, Hong SK, Lee JM, et al. 2021; No diffuse intrahepatic biliary stricture after ABO-incompatible adult living donor liver transplantation using tailored rituximab-based desensitization protocol. Ann Transl Med. 9:30. DOI: 10.21037/atm-20-4703. PMID: 33553323. PMCID: PMC7859775.6. Egawa H. 2020; Challenge to ABO blood type barrier in living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 19:342–8. DOI: 10.1016/j.hbpd.2020.06.017. PMID: 32665181.7. Kessel A, Rosner I, Toubi E. 2008; Rituximab: beyond simple B cell depletion. Clin Rev Allergy Immunol. 34:74–9. DOI: 10.1007/s12016-008-8074-1. PMID: 18240027.8. Sakai H, Tanaka Y, Tazawa H, Shimizu S, Verma S, Ohira M, et al. 2017; Effect of Fc-γ receptor polymorphism on rituximab-mediated B cell depletion in ABO-incompatible adult living donor liver transplantation. Transplant Direct. 3:e164. DOI: 10.1097/TXD.0000000000000683. PMID: 28620648. PMCID: PMC5464783.9. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. 2002; Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 99:754–8. DOI: 10.1182/blood.V99.3.754. PMID: 11806974.10. Liu F, Ding H, Jin X, Ding N, Deng L, He Y, et al. 2014; FCGR3A 158V/F polymorphism and response to frontline R-CHOP therapy in diffuse large B-cell lymphoma. DNA Cell Biol. 33:616–23. DOI: 10.1089/dna.2013.2333. PMID: 25050883. PMCID: PMC4144364.11. Das LK, Ide K, Tanaka A, Morimoto H, Shimizu S, Tanimine N, et al. 2017; Fc-gamma receptor 3A polymorphism predicts the incidence of urinary tract infection in kidney-transplant recipients. Hum Immunol. 78:357–62. DOI: 10.1016/j.humimm.2017.03.006. PMID: 28315348.12. Paul P, Pedini P, Lyonnet L, Di Cristofaro J, Loundou A, Pelardy M, et al. 2019; FCGR3A and FCGR2A genotypes differentially impact allograft rejection and patients' survival after lung transplant. Front Immunol. 10:1208. DOI: 10.3389/fimmu.2019.01208. PMID: 31249568. PMCID: PMC6582937.13. Shimizu S, Tanaka Y, Tazawa H, Verma S, Onoe T, Ishiyama K, et al. 2016; Fc-gamma receptor polymorphisms predispose patients to infectious complications after liver transplantation. Am J Transplant. 16:625–33. DOI: 10.1111/ajt.13492. PMID: 26517570.14. Rummler S, Bauschke A, Baerthel E, Juette H, Maier K, Malessa C, et al. 2017; ABO-incompatible living donor liver transplantation in focus of antibody rebound. Transfus Med Hemother. 44:46–51. DOI: 10.1159/000450792. PMID: 28275333. PMCID: PMC5318927.15. Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, et al. 2014; Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 61:575–82. DOI: 10.1016/j.jhep.2014.04.039. PMID: 24801413.16. Ishida H, Kondo T, Shimizu T, Nozaki T, Tanabe K. 2015; Postoperative rebound of antiblood type antibodies and antibody-mediated rejection after ABO-incompatible living-related kidney transplantation. Transpl Int. 28:286–96. DOI: 10.1111/tri.12482. PMID: 25363583.17. Hussain K, Hargreaves CE, Rowley TF, Sopp JM, Latham KV, Bhatta P, et al. 2019; Impact of human FcγR gene polymorphisms on IgG-triggered cytokine release: critical importance of cell assay format. Front Immunol. 10:390. DOI: 10.3389/fimmu.2019.00390. PMID: 30899264. PMCID: PMC6417454.18. Sarmiento E, Cifrian J, Calahorra L, Bravo C, Lopez S, Laporta R, et al. 2018; Monitoring of early humoral immunity to identify lung recipients at risk for development of serious infections: a multicenter prospective study. J Heart Lung Transplant. 37:1001–12. DOI: 10.1016/j.healun.2018.04.001. PMID: 29754764.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO-Incompatible Living Donor Liver Transplantation
- Overcoming high pre-transplant isoagglutinin titers using high-dose intravenous immunoglobulin, salvage plasmapheresis, and booster rituximab without splenectomy in ABO-incompatible living donor liver transplantation: a case report
- Immunologic strategies and outcomes in ABO-incompatible living donor liver transplantation
- ABO-incompatible living donor liver transplantation with a simplified desensitization and immunosuppression protocol: a single center retrospective study
- ABO Incompatible Living Donor Liver Transplantation: A Single Center Experience