Diabetes Metab J.
2023 Sep;47(5):653-667. 10.4093/dmj.2022.0244.
CycloZ Improves Hyperglycemia and Lipid Metabolism by Modulating Lysine Acetylation in KK-Ay Mice
- Affiliations
-
- 1R&D Center, NovMetaPharma Co., Ltd., Seoul, Korea
- 2Department of Life Sciences, Pohang University of Science and Technology (POSTECH), Pohang, Korea
- 3Research Institute of Aging and Metabolism, Kyungpook National University, Daegu, Korea
- 4Department of Internal Medicine, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 5School of Life Science, Handong Global University, Pohang, Korea
- 6Department of Medicine, Graduate School, Daegu Catholic University, Gyeongsan, Korea
- 7Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon, Korea
- 8Biomedical Institute for Convergence at SKKU (BICS), Sungkyunkwan University, Suwon, Korea
- 9Graduate School of Medical Science, Brain Korea 21 Project, Yonsei University College of Medicine, Seoul, Korea
- 10Severance Biomedical Science Institute, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 11Laboratory of Integrative Systems Physiology, Institute of Bioengineering, Swiss Federal Institute of Technology in Lausanne, Lausanne, Switzerland
- 12School of Interdisciplinary Bioscience and Bioengineering, Pohang University of Science and Technology (POSTECH), Pohang, Korea
- KMID: 2546124
- DOI: http://doi.org/10.4093/dmj.2022.0244
Abstract
- Background
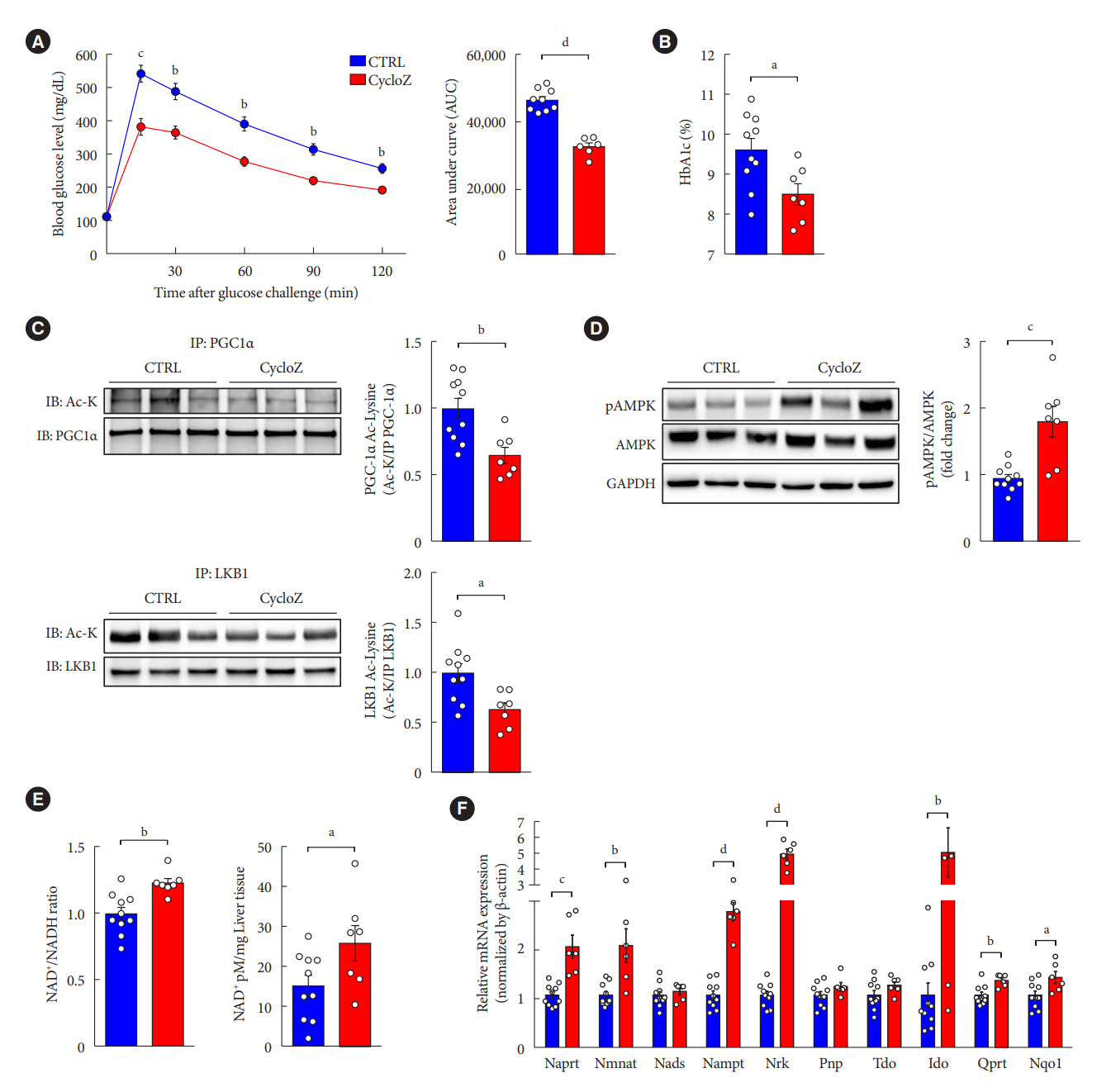
CycloZ, a combination of cyclo-His-Pro and zinc, has anti-diabetic activity. However, its exact mode of action remains to be elucidated.
Methods
KK-Ay mice, a type 2 diabetes mellitus (T2DM) model, were administered CycloZ either as a preventive intervention, or as a therapy. Glycemic control was evaluated using the oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) levels. Liver and visceral adipose tissues (VATs) were used for histological evaluation, gene expression analysis, and protein expression analysis.
Results
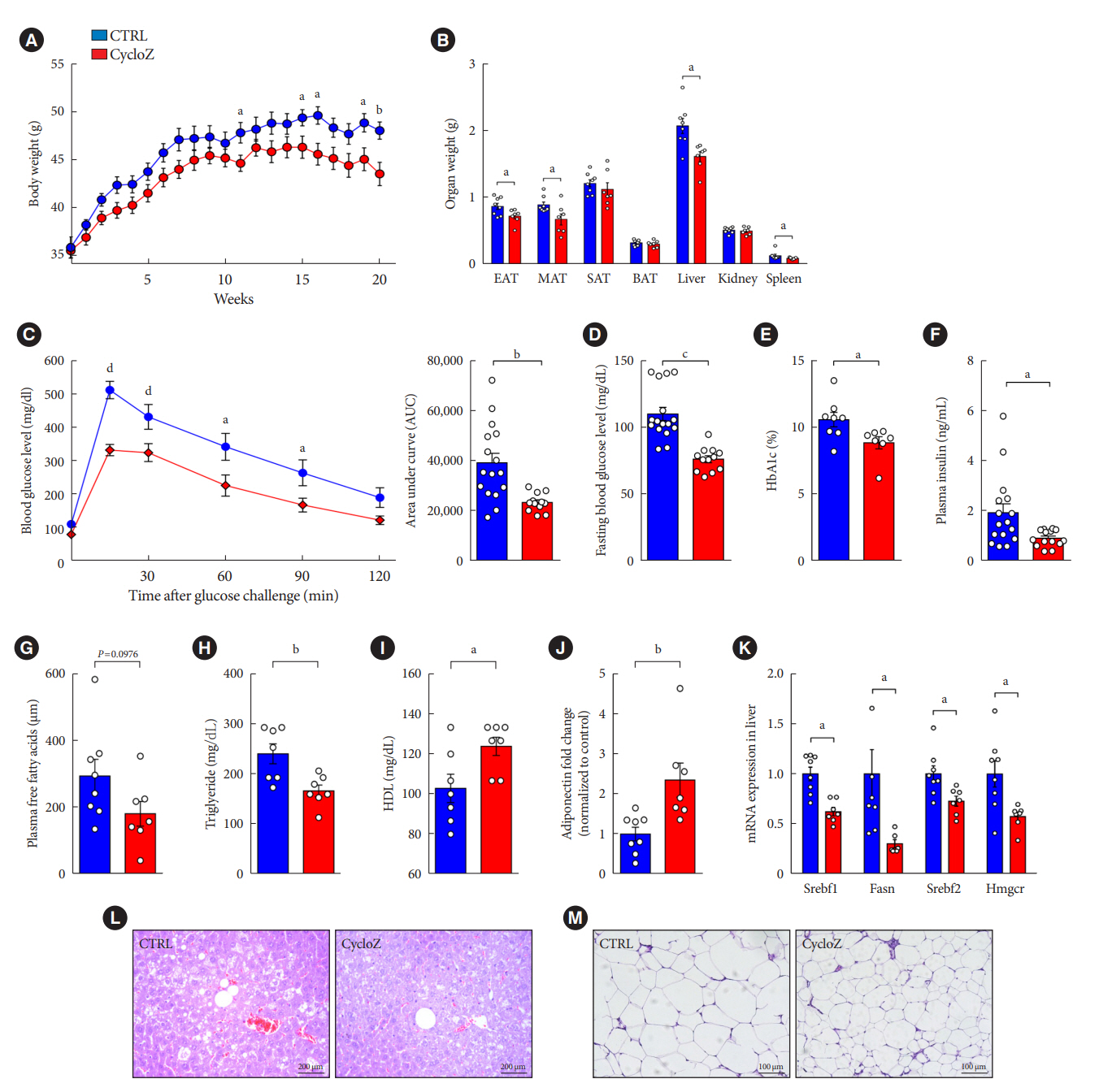
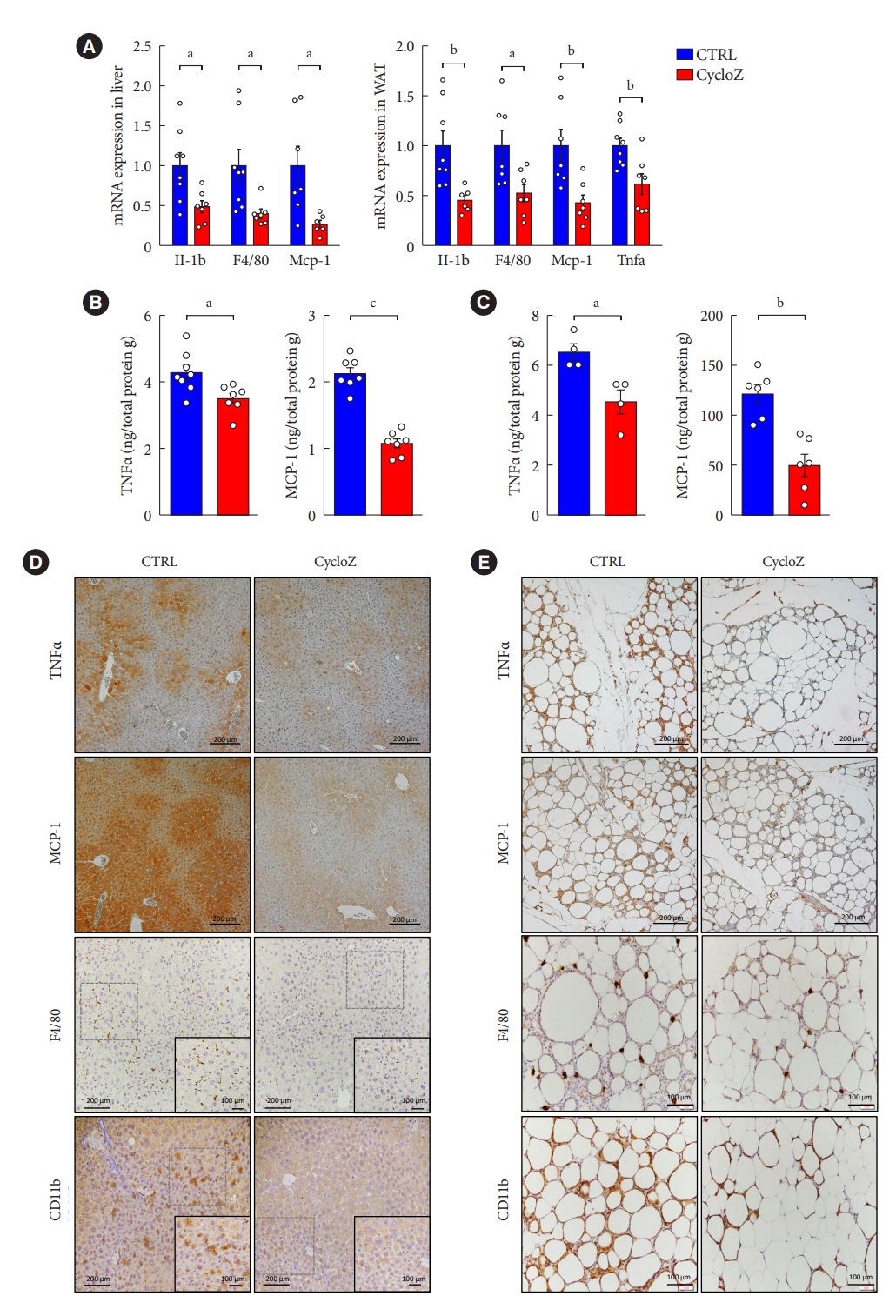
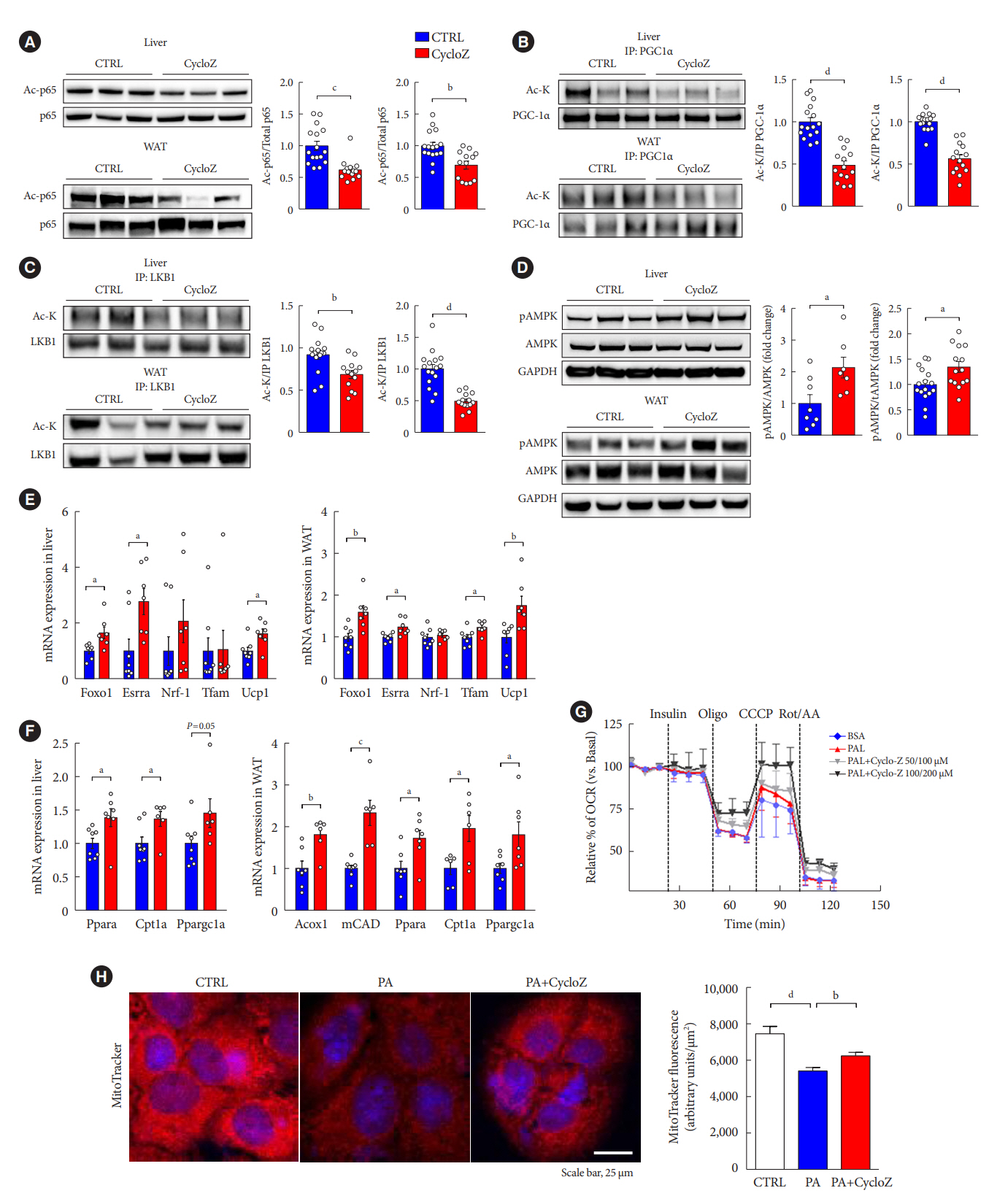
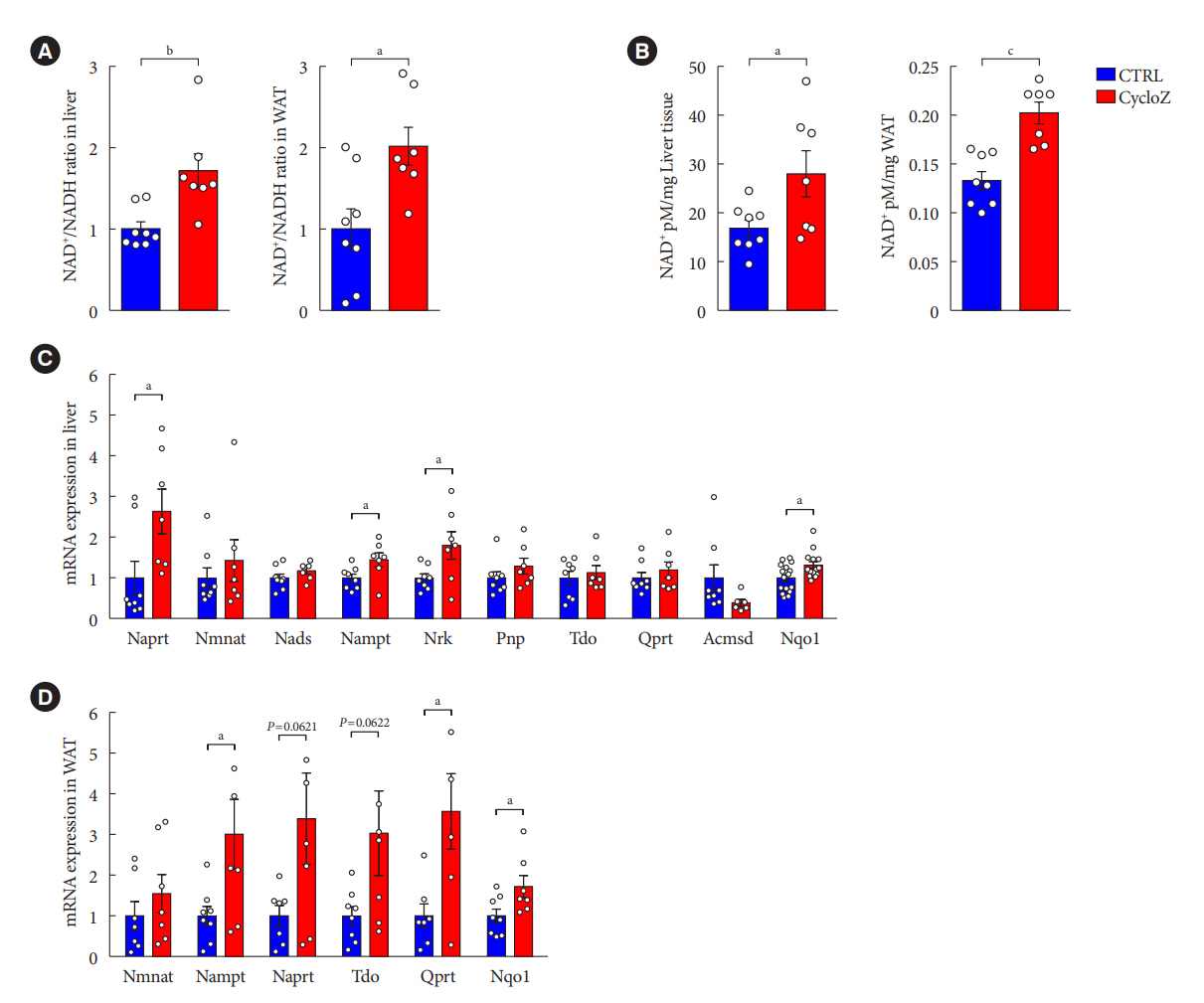
CycloZ administration improved glycemic control in KK-Ay mice in both prophylactic and therapeutic studies. Lysine acetylation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, liver kinase B1, and nuclear factor-κB p65 was decreased in the liver and VATs in CycloZ-treated mice. In addition, CycloZ treatment improved mitochondrial function, lipid oxidation, and inflammation in the liver and VATs of mice. CycloZ treatment also increased the level of β-nicotinamide adenine dinucleotide (NAD+), which affected the activity of deacetylases, such as sirtuin 1 (Sirt1).
Conclusion
Our findings suggest that the beneficial effects of CycloZ on diabetes and obesity occur through increased NAD+ synthesis, which modulates Sirt1 deacetylase activity in the liver and VATs. Given that the mode of action of an NAD+ booster or Sirt1 deacetylase activator is different from that of traditional T2DM drugs, CycloZ would be considered a novel therapeutic option for the treatment of T2DM.
Keyword
Figure
Reference
-
1. Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020; 21:6275.2. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41:2669–701.3. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care. 2018; 41:69–78.4. White JR Jr. A brief history of the development of diabetes medications. Diabetes Spectr. 2014; 27:82–6.5. Freeland B, Farber MS. Type 2 diabetes drugs: a review. Home Healthc Now. 2015; 33:304–10.6. Pathak R, Bridgeman MB. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. P T. 2010; 35:509–13.7. Garofalo C, Borrelli S, Liberti ME, Andreucci M, Conte G, Minutolo R, et al. SGLT2 inhibitors: nephroprotective efficacy and side effects. Medicina (Kaunas). 2019; 55:268.8. Norvell A, McMahon SB. Cell biology: rise of the rival. Science. 2010; 327:964–5.9. Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010; 327:1004–7.10. Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13:225–38.11. Menzies KJ, Zhang H, Katsyuba E, Auwerx J. Protein acetylation in metabolism: metabolites and cofactors. Nat Rev Endocrinol. 2016; 12:43–60.12. Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009; 20:98–105.13. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008; 283:27628–35.14. Vannini N, Campos V, Girotra M, Trachsel V, Rojas-Sutterlin S, Tratwal J, et al. The NAD-booster nicotinamide riboside potently stimulates hematopoiesis through increased mitochondrial clearance. Cell Stem Cell. 2019; 24:405–18.15. Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016; 352:1436–43.16. Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 2018; 27:529–47.17. Wang YW, Sun GD, Sun J, Liu SJ, Wang J, Xu XH, et al. Spontaneous type 2 diabetic rodent models. J Diabetes Res. 2013; 2013:401723.18. Iwatsuka H, Shino A, Suzuoki Z. General survey of diabetic features of yellow KK mice. Endocrinol Jpn. 1970; 17:23–35.19. Minelli A, Grottelli S, Mierla A, Pinnen F, Cacciatore I, Bellezza I. Cyclo(His-Pro) exerts anti-inflammatory effects by modulating NF-κB and Nrf2 signalling. Int J Biochem Cell Biol. 2012; 44:525–35.20. Mizuma T, Masubuchi S, Awazu S. Intestinal absorption of stable cyclic glycylphenylalanine: comparison with the linear form. J Pharm Pharmacol. 1997; 49:1067–71.21. Jaspan JB, Banks WA, Kastin AJ. Study of passage of peptides across the blood-brain barrier: biological effects of cyclo(His-Pro) after intravenous and oral administration. Ann N Y Acad Sci. 1994; 739:101–7.22. Prasad C. Cyclo(His-Pro): its distribution, origin and function in the human. Neurosci Biobehav Rev. 1988; 12:19–22.23. Song MK, Hwang IK, Rosenthal MJ, Harris DM, Yamaguchi DT, Yip I, et al. Anti-hyperglycemic activity of zinc plus cyclo (his-pro) in genetically diabetic Goto-Kakizaki and aged rats. Exp Biol Med (Maywood). 2003; 228:1338–45.24. Song MK, Rosenthal MJ, Song AM, Uyemura K, Yang H, Ament ME, et al. Body weight reduction in rats by oral treatment with zinc plus cyclo-(His-Pro). Br J Pharmacol. 2009; 158:442–50.25. Hwang IK, Go VL, Harris DM, Yip I, Kang KW, Song MK. Effects of cyclo (his-pro) plus zinc on glucose metabolism in genetically diabetic obese mice. Diabetes Obes Metab. 2003; 5:317–24.26. Jung HY, Kim B, Ryu HG, Ji Y, Park S, Choi SH, et al. Amodiaquine improves insulin resistance and lipid metabolism in diabetic model mice. Diabetes Obes Metab. 2018; 20:1688–701.27. Kim HJ, Kim JY, Lee SJ, Kim HJ, Oh CJ, Choi YK, et al. α-Lipoic acid prevents neointimal hyperplasia via induction of p38 mitogen-activated protein kinase/Nur77-mediated apoptosis of vascular smooth muscle cells and accelerates postinjury reendothelialization. Arterioscler Thromb Vasc Biol. 2010; 30:2164–72.28. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004; 23:2369–80.29. Berthiaume JM, Hsiung CH, Austin AB, McBrayer SP, Depuydt MM, Chandler MP, et al. Methylene blue decreases mitochondrial lysine acetylation in the diabetic heart. Mol Cell Biochem. 2017; 432:7–24.30. Kosanam H, Thai K, Zhang Y, Advani A, Connelly KA, Diamandis EP, et al. Diabetes induces lysine acetylation of intermediary metabolism enzymes in the kidney. Diabetes. 2014; 63:2432–9.31. Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009; 460:587–91.32. Katsyuba E, Romani M, Hofer D, Auwerx J. NAD+ homeostasis in health and disease. Nat Metab. 2020; 2:9–31.33. Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983; 75:273–7.34. Failla ML, Gardell CY. Influence of spontaneous diabetes on tissue status of zinc, copper, and manganese in the BB Wistar rat. Proc Soc Exp Biol Med. 1985; 180:317–22.35. Zhao Y, Tan Y, Dai J, Wang B, Li B, Guo L, et al. Zinc deficiency exacerbates diabetic down-regulation of Akt expression and function in the testis: essential roles of PTEN, PTP1B and TRB3. J Nutr Biochem. 2012; 23:1018–26.36. Rosenthal MJ, Hwang IK, Song MK. Effects of arachidonic acid and cyclo (his-pro) on zinc transport across small intestine and muscle tissues. Life Sci. 2001; 70:337–48.37. Song MK, Bischoff DS, Song AM, Uyemura K, Yamaguchi DT. Metabolic relationship between diabetes and Alzheimer’s Disease affected by Cyclo(His-Pro) plus zinc treatment. BBA Clin. 2016; 7:41–54.38. Tang WJ. Targeting insulin-degrading enzyme to treat type 2 diabetes mellitus. Trends Endocrinol Metab. 2016; 27:24–34.39. El Dib R, Gameiro OL, Ogata MS, Modolo NS, Braz LG, Jorge EC, et al. Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane Database Syst Rev. 2015; 5:CD005525.40. Jayawardena R, Ranasinghe P, Galappatthy P, Malkanthi R, Constantine G, Katulanda P. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2012; 4:13.41. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007; 116:39–48.42. Hara K, Tobe K, Okada T, Kadowaki H, Akanuma Y, Ito C, et al. A genetic variation in the PGC-1 gene could confer insulin resistance and susceptibility to type II diabetes. Diabetologia. 2002; 45:740–3.43. Muller YL, Bogardus C, Pedersen O, Baier L. A Gly482Ser missense mutation in the peroxisome proliferator-activated receptor gamma coactivator-1 is associated with altered lipid oxidation and early insulin secretion in Pima Indians. Diabetes. 2003; 52:895–8.44. Hammarstedt A, Jansson PA, Wesslau C, Yang X, Smith U. Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys Res Commun. 2003; 301:578–82.45. Grottelli S, Mezzasoma L, Scarpelli P, Cacciatore I, Cellini B, Bellezza I. Cyclo(His-Pro) inhibits NLRP3 inflammasome cascade in ALS microglial cells. Mol Cell Neurosci. 2019; 94:23–31.46. Li XQ, Lei J, Mao LH, Wang QL, Xu F, Ran T, et al. NAMPT and NAPRT, key enzymes in NAD salvage synthesis pathway, are of negative prognostic value in colorectal cancer. Front Oncol. 2019; 9:736.47. Sacitharan PK, Bou-Gharios G, Edwards JR. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Discov. 2020; 6:41.48. Ling L, Gu S, Cheng Y. Resveratrol inhibits adventitial fibroblast proliferation and induces cell apoptosis through the SIRT1 pathway. Mol Med Rep. 2017; 15:567–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Free fatty acid-induced histone acetyltransferase activity accelerates lipid accumulation in HepG2 cells
- Post-Translational Modification of Proteins in Toxicological Research: Focus on Lysine Acylation
- Supplementary Effect of gamma-Oryzanol on Lipid Metabolism in Diabetic KK Mice
- Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity
- Effect of Lysine -Limited Diets Containing Different Levels of L -Carnitine on Body Weight and Lipid Metabolism in Obesity -Induced Adult Rats