Anat Cell Biol.
2016 Sep;49(3):165-176. 10.5115/acb.2016.49.3.165.
Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity
- Affiliations
-
- 1Department of Anatomy, Jeju National University School of Medicine, Jeju, Korea. jinu.kim@jejunu.ac.kr
- 2Department of Biomedicine and Drug Development, Jeju National University, Jeju, Korea.
- KMID: 2353341
- DOI: http://doi.org/10.5115/acb.2016.49.3.165
Abstract
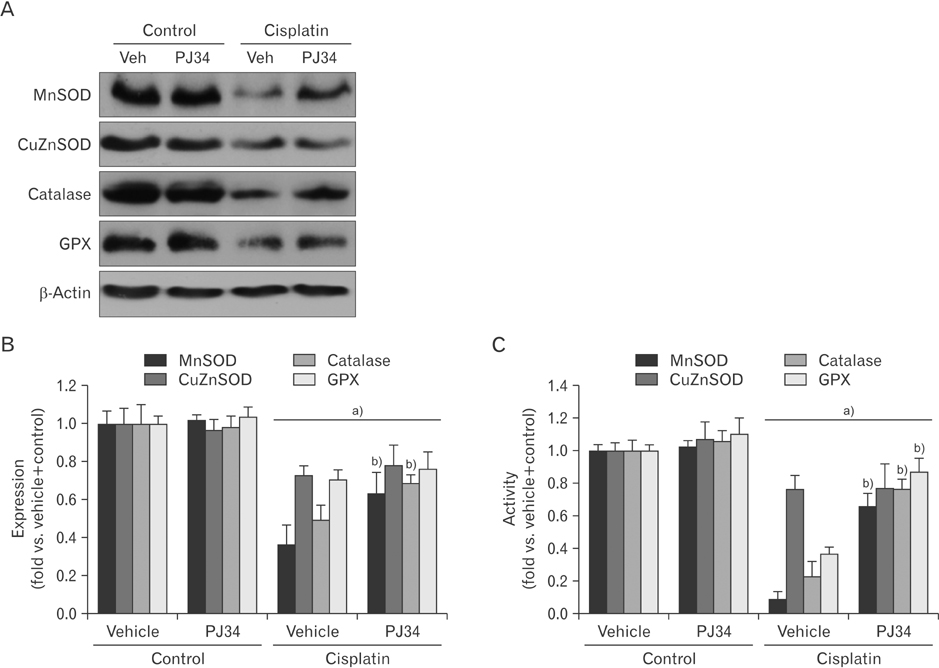
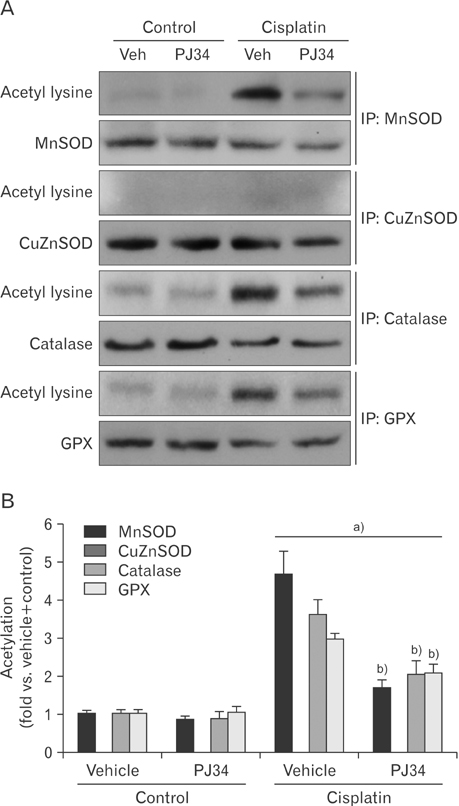
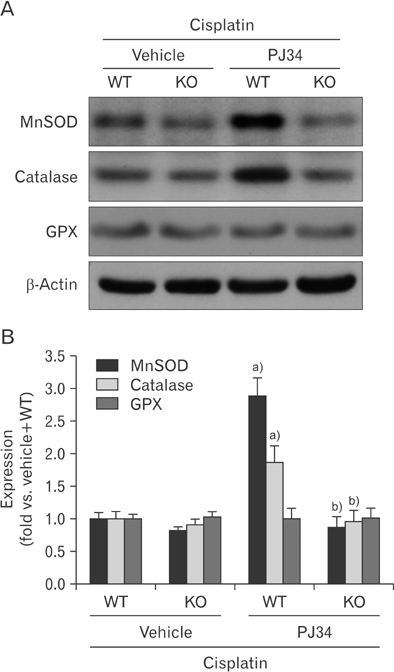
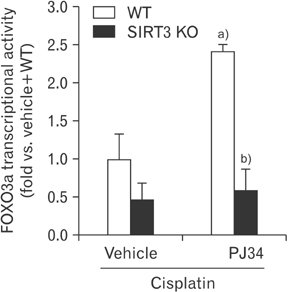
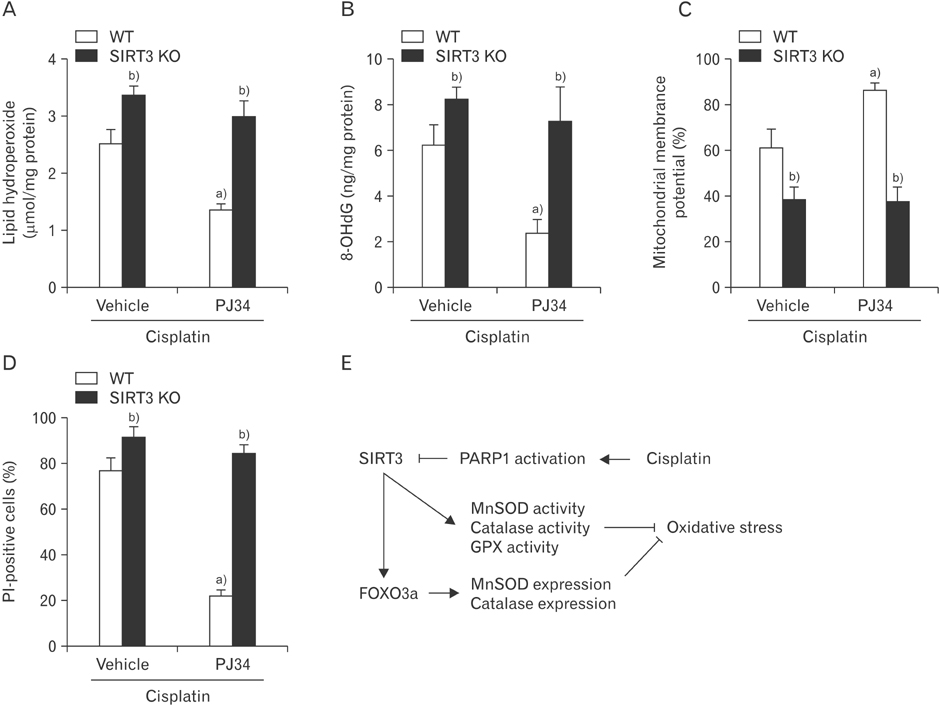
- Enhanced oxidative stress is a hallmark of cisplatin nephrotoxicity, and inhibition of poly(ADP-ribose) polymerase 1 (PARP1) attenuates oxidative stress during cisplatin nephrotoxicity; however, the precise mechanisms behind its action remain elusive. Here, using an in vitro model of cisplatin-induced injury to human kidney proximal tubular cells, we demonstrated that the protective effect of PARP1 inhibition on oxidative stress is associated with sirtuin 3 (SIRT3) activation. Exposure to 400 µM cisplatin for 8 hours in cells decreased activity and expression of manganese superoxide dismutase (MnSOD), catalase, glutathione peroxidase (GPX), and SIRT3, while it increased their lysine acetylation. However, treatment with 1 µM PJ34 hydrochloride, a potent PARP1 inhibitor, restored activity and/or expression in those antioxidant enzymes, decreased lysine acetylation of those enzymes, and improved SIRT3 expression and activity in the cisplatin-injured cells. Using transfection with SIRT3 double nickase plasmids, SIRT3-deficient cells given cisplatin did not show the ameliorable effect of PARP1 inhibition on lysine acetylation and activity of antioxidant enzymes, including MnSOD, catalase and GPX. Furthermore, SIRT3 deficiency in cisplatin-injured cells prevented PARP1 inhibition-induced increase in forkhead box O3a transcriptional activity, and upregulation of MnSOD and catalase. Finally, loss of SIRT3 in cisplatin-exposed cells removed the protective effect of PARP1 inhibition against oxidative stress, represented by the concentration of lipid hydroperoxide and 8-hydroxy-2'-deoxyguanosine; and necrotic cell death represented by a percentage of propidium iodide-positively stained cells. Taken together, these results indicate that PARP1 inhibition protects kidney proximal tubular cells against oxidative stress through SIRT3 activation during cisplatin nephrotoxicity.
Keyword
MeSH Terms
-
Acetylation
Catalase
Cell Death
Cisplatin*
Deoxyribonuclease I
Down-Regulation*
Glutathione Peroxidase
Humans
In Vitro Techniques
Kidney
Lipid Peroxides
Lysine
Oxidative Stress*
Plasmids
Poly Adenosine Diphosphate Ribose*
Poly(ADP-ribose) Polymerases*
Propidium
Sirtuin 3*
Superoxide Dismutase
Transfection
Up-Regulation
Catalase
Cisplatin
Deoxyribonuclease I
Glutathione Peroxidase
Lipid Peroxides
Lysine
Poly Adenosine Diphosphate Ribose
Poly(ADP-ribose) Polymerases
Propidium
Sirtuin 3
Superoxide Dismutase
Figure
Cited by 2 articles
-
Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity
Sang Pil Yoon, Jinu Kim
Anat Cell Biol. 2018;51(3):189-199. doi: 10.5115/acb.2018.51.3.189.Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
Daeun Moon, Jinu Kim
Anat Cell Biol. 2019;52(3):312-323. doi: 10.5115/acb.18.192.
Reference
-
1. Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008; 73:994–1007.2. Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Solution structure of a cisplatin-induced DNA interstrand cross-link. Science. 1995; 270:1842–1845.3. Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003; 23:460–464.4. Santos NA, Catao CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007; 81:495–504.5. Somani SM, Husain K, Whitworth C, Trammell GL, Malafa M, Rybak LP. Dose-dependent protection by lipoic acid against cisplatin-induced nephrotoxicity in rats: antioxidant defense system. Pharmacol Toxicol. 2000; 86:234–241.6. Krishnakumar R, Kraus WL. The PARP side of the nucleus: molecular actions, physiological outcomes, and clinical targets. Mol Cell. 2010; 39:8–24.7. Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999; 96:13978–13982.8. Martin DR, Lewington AJ, Hammerman MR, Padanilam BJ. Inhibition of poly(ADP-ribose) polymerase attenuates ischemic renal injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000; 279:R1834–R1840.9. Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011; 301:F450–F459.10. Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 activation links ischemic acute kidney injury to interstitial fibrosis. J Physiol Sci. 2015; 65:105–111.11. Shevalye H, Maksimchyk Y, Watcho P, Obrosova IG. Poly(ADP-ribose) polymerase-1 (PARP-1) gene deficiency alleviates diabetic kidney disease. Biochim Biophys Acta. 2010; 1802:1020–1027.12. Jog NR, Dinnall JA, Gallucci S, Madaio MP, Caricchio R. Poly(ADP-ribose) polymerase-1 regulates the progression of autoimmune nephritis in males by inducing necrotic cell death and modulating inflammation. J Immunol. 2009; 182:7297–7306.13. Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012; 82:193–203.14. Kim J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol Res. 2016; 65:333–340.15. Park S, Yoon SP, Kim J. Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol. 2015; 48:66–74.16. Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008; 173:2–13.17. Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011; 12:534–541.18. Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E, Gibson BW. Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci U S A. 2013; 110:6601–6606.19. Zhou X, Chen M, Zeng X, Yang J, Deng H, Yi L, Mi MT. Resveratrol regulates mitochondrial reactive oxygen species homeostasis through Sirt3 signaling pathway in human vascular endothelial cells. Cell Death Dis. 2014; 5:e1576.20. Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012; 287:14078–14086.21. Chen CJ, Fu YC, Yu W, Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem Biophys Res Commun. 2013; 430:798–803.22. Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009; 119:2758–2771.23. Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013; 63:222–234.24. Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, Novelli R, Remuzzi G, Benigni A. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015; 125:715–726.25. Gao J, Zheng Z, Gu Q, Chen X, Liu X, Xu X. Deacetylation of MnSOD by PARP-regulated SIRT3 protects retinal capillary endothelial cells from hyperglycemia-induced damage. Biochem Biophys Res Commun. 2016; 472:425–431.26. Kim J, Kim KY, Jang HS, Yoshida T, Tsuchiya K, Nitta K, Park JW, Bonventre JV, Park KM. Role of cytosolic NADP+-dependent isocitrate dehydrogenase in ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2009; 296:F622–F633.27. Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007; 2:287–295.28. Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice: a role for manganese superoxide dismutase. J Biol Chem. 2006; 281:20349–20356.29. Song H, Yoon SP, Kim J. Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells. Anat Cell Biol. 2016; 49:79–87.30. Xiong Y, Guan KL. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J Cell Biol. 2012; 198:155–164.31. Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010; 40:893–904.32. Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012; 92:1479–1514.33. Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011; 13:461–468.34. Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci U S A. 2004; 101:10042–10047.35. Tan WQ, Wang K, Lv DY, Li PF. Foxo3a inhibits cardiomyocyte hypertrophy through transactivating catalase. J Biol Chem. 2008; 283:29730–29739.36. Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005; 4:421–440.37. Szabó C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998; 19:287–298.38. Fassett RG, D'Intini V, Healy H, Gowardman J, Gan JS, Sharman JE, Coombes JS. Assessment of arterial stiffness, oxidative stress and inflammation in acute kidney injury. BMC Nephrol. 2009; 10:15.39. Krishnakumar R, Kraus WL. PARP-1 regulates chromatin structure and transcription through a KDM5B-dependent pathway. Mol Cell. 2010; 39:736–749.40. Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002; 419:316–321.41. Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, Sueyasu K, Washida N, Tokuyama H, Tzukerman M, Skorecki K, Hayashi K, Itoh H. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. J Biol Chem. 2010; 285:13045–13056.42. Mishima K, Baba A, Matsuo M, Itoh Y, Oishi R. Protective effect of cyclic AMP against cisplatin-induced nephrotoxicity. Free Radic Biol Med. 2006; 40:1564–1577.43. Sadzuka Y, Shoji T, Takino Y. Effect of cisplatin on the activities of enzymes which protect against lipid peroxidation. Biochem Pharmacol. 1992; 43:1872–1875.44. Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009; 61:223–242.45. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003; 22:7265–7279.46. Kruidering M, Van de Water B, de Heer E, Mulder GJ, Nagelkerke JF. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997; 280:638–649.47. Liu H, Baliga R. Cytochrome P450 2E1 null mice provide novel protection against cisplatin-induced nephrotoxicity and apoptosis. Kidney Int. 2003; 63:1687–1696.48. Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001; 12:2683–2690.49. Ahmed LA, Shehata NI, Abdelkader NF, Khattab MM. Tempol, a superoxide dismutase mimetic agent, ameliorates cisplatin-induced nephrotoxicity through alleviation of mitochondrial dysfunction in mice. PLoS One. 2014; 9:e108889.50. Bause AS, Haigis MC. SIRT3 regulation of mitochondrial oxidative stress. Exp Gerontol. 2013; 48:634–639.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells
- Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity
- Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation
- Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies
- The Important Role of Poly ADP-Ribose Polymerase Inhibitor in Prostate Cancer