Nutr Res Pract.
2023 Aug;17(4):631-640. 10.4162/nrp.2023.17.4.631.
Effects of coffee intake on airway hypersensitivity and immunomodulation: an in vivo murine study
- Affiliations
-
- 1Department of Pediatrics, Taipei Veterans General Hospital, Taipei 114, Taiwan
- 2Department of Pediatrics, Taipei Medical University Hospital, Taipei 110, Taiwan
- 3Department of Pediatrics, Tri-Service General Hospital, Taipei 114, Taiwan
- 4School of Medicine, National Defense Medical Center, Taipei 114, Taiwan
- KMID: 2545181
- DOI: http://doi.org/10.4162/nrp.2023.17.4.631
Abstract
- BACKGROUND/OBJECTIVES
Coffee is a complex chemical mixture, with caffeine being the most well-known bioactive substance. The immunomodulatory and anti-inflammatory properties of coffee and caffeine impact health in various aspects, including the respiratory system. The objective is to investigate the effects of coffee and caffeine on airway hyperresponsiveness and allergic reactions, as well as to analyze and compare associated cytokine profiles.
MATERIALS/METHODS
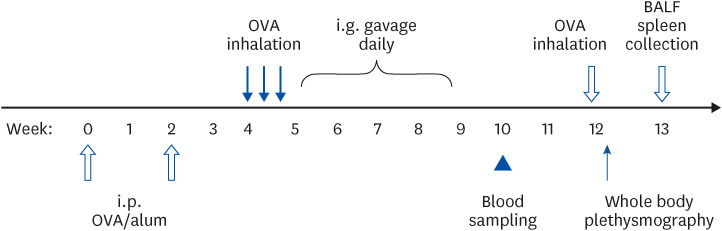
BALB/c mice were intraperitoneally sensitized with ovalbumin (OVA) and given OVA inhalation to induce airway hypersensitivity. Two weeks after sensitization, they were intragastrically gavaged with coffee or caffeine, both containing 0.3125 mg caffeine, daily for 4 weeks. Control mice were fed with double-distilled water. Serum OVAspecific antibody levels were measured beforehand and 5 weeks after the first gavage. Airway hyperresponsiveness was detected by whole body plethysmography after gavage. Cytokine levels of bronchoalveolar lavage and cultured splenocytes were analyzed.
RESULTS
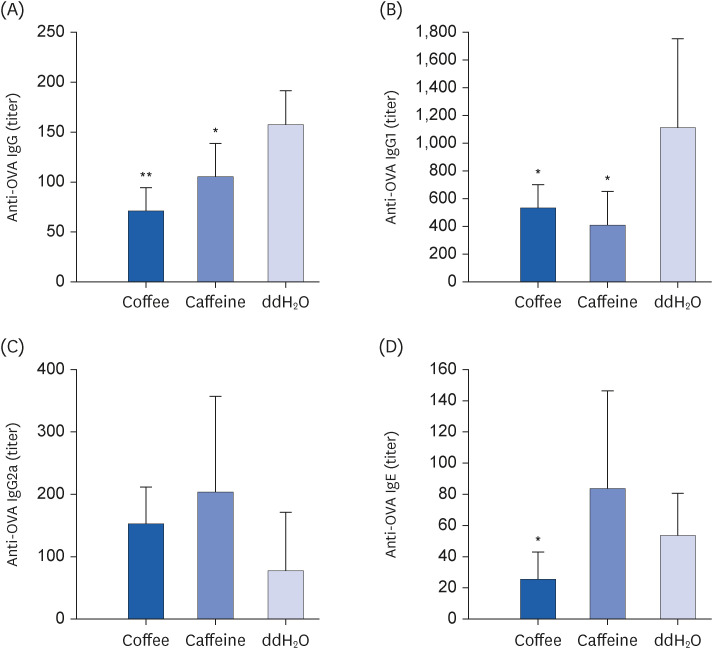
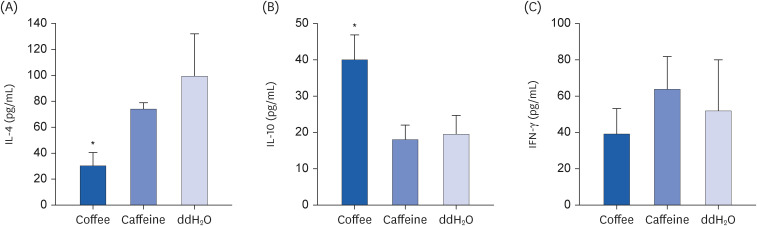
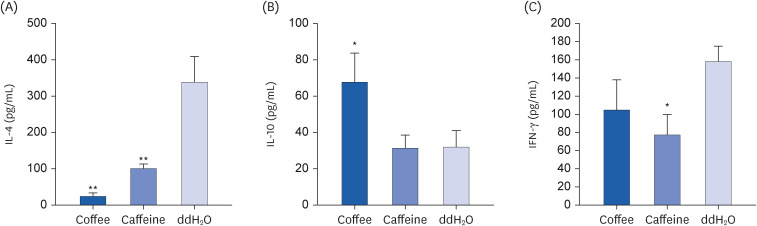
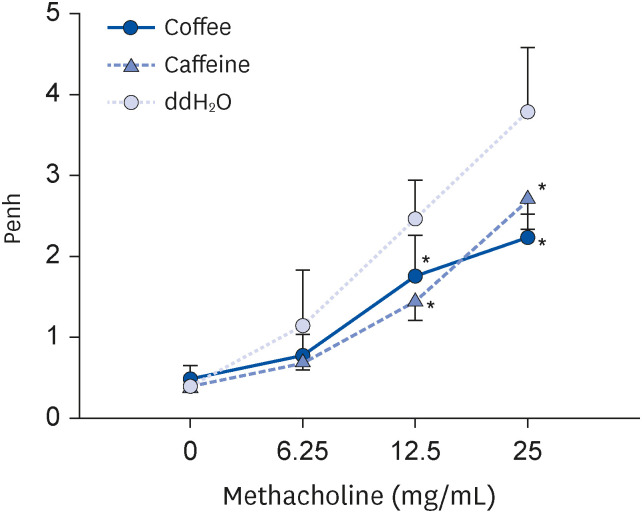
Coffee effectively suppressed T helper 2-mediated specific antibody response. Airway responsiveness was reduced in mice treated with either coffee or caffeine. Compared to the control, coffee significantly reduced OVA-specific immunoglobulin (Ig) G, IgG1 and IgE antibody responses (P < 0.05). Caffeine also attenuated specific IgG and IgG1 levels, though IgE level was unaffected. Coffee significantly reduced interleukin (IL)-4 and increased IL-10 concentration in spleen cells and bronchoalveolar lavage fluid (P < 0.05).
CONCLUSIONS
Coffee effectively attenuated airway hyperresponsiveness and systemic allergic responses induced by OVA food allergen in mice. As a complex composition of bioactive substances, coffee displayed enhanced immunomodulatory and anti-inflammatory effects than caffeine.
Figure
Reference
-
1. Jeszka-Skowron M, Zgoła-Grześkowiak A, Grześkowiak T. Analytical methods applied for the characterization and the determination of bioactive compounds in coffee. Eur Food Res Technol. 2015; 240:19–31.
Article2. Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017; 359:j5024. PMID: 29167102.
Article3. Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev. 2017; 26:368–377. PMID: 27111112.
Article4. Caini S, Cattaruzza MS, Bendinelli B, Tosti G, Masala G, Gnagnarella P, Assedi M, Stanganelli I, Palli D, Gandini S. Coffee, tea and caffeine intake and the risk of non-melanoma skin cancer: a review of the literature and meta-analysis. Eur J Nutr. 2017; 56:1–12.
Article5. Johnson S, Koh WP, Wang R, Govindarajan S, Yu MC, Yuan JM. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes Control. 2011; 22:503–510. PMID: 21258859.
Article6. Liu H, Hu GH, Wang XC, Huang TB, Xu L, Lai P, Guo ZF, Xu YF. Coffee consumption and prostate cancer risk: a meta-analysis of cohort studies. Nutr Cancer. 2015; 67:392–400. PMID: 25706900.
Article7. Wang A, Wang S, Zhu C, Huang H, Wu L, Wan X, Yang X, Zhang H, Miao R, He L, et al. Coffee and cancer risk: a meta-analysis of prospective observational studies. Sci Rep. 2016; 6:33711. PMID: 27665923.
Article8. Zhou Q, Luo ML, Li H, Li M, Zhou JG. Coffee consumption and risk of endometrial cancer: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015; 5:13410. PMID: 26302813.
Article9. Wijarnpreecha K, Thongprayoon C, Ungprasert P. Coffee consumption and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017; 29:e8–12. PMID: 27824642.
Article10. Zhang YP, Li WQ, Sun YL, Zhu RT, Wang WJ. Systematic review with meta-analysis: coffee consumption and the risk of gallstone disease. Aliment Pharmacol Ther. 2015; 42:637–648. PMID: 26198295.
Article11. Liu F, Wang X, Wu G, Chen L, Hu P, Ren H, Hu H. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015; 10:e0142457. PMID: 26556483.
Article12. Baspinar B, Eskici G, Ozcelik AO. How coffee affects metabolic syndrome and its components. Food Funct. 2017; 8:2089–2101. PMID: 28589997.
Article13. Grosso G, Micek A, Godos J, Sciacca S, Pajak A, Martínez-González MA, Giovannucci EL, Galvano F. Coffee consumption and risk of all-cause, cardiovascular, and cancer mortality in smokers and non-smokers: a dose-response meta-analysis. Eur J Epidemiol. 2016; 31:1191–1205. PMID: 27699514.
Article14. Rodríguez-Artalejo F, López-García E. Coffee consumption and cardiovascular disease: a condensed review of epidemiological evidence and mechanisms. J Agric Food Chem. 2018; 66:5257–5263. PMID: 29276945.
Article15. Wang L, Shen X, Wu Y, Zhang D. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016; 50:228–242. PMID: 26339067.
Article16. Grosso G, Micek A, Castellano S, Pajak A, Galvano F. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016; 60:223–234. PMID: 26518745.
Article17. Park KY, Kim HJ, Ahn HS, Kim SH, Park EJ, Yim SY, Jun JB. Effects of coffee consumption on serum uric acid: systematic review and meta-analysis. Semin Arthritis Rheum. 2016; 45:580–586. PMID: 26905267.
Article18. Peerapen P, Thongboonkerd V. Caffeine in kidney stone disease: risk or benefit? Adv Nutr. 2018; 9:419–424. PMID: 30032225.
Article19. Alfaro TM, Monteiro RA, Cunha RA, Cordeiro CR. Chronic coffee consumption and respiratory disease: a systematic review. Clin Respir J. 2018; 12:1283–1294. PMID: 28671769.
Article20. Oñatibia-Astibia A, Martínez-Pinilla E, Franco R. The potential of methylxanthine-based therapies in pediatric respiratory tract diseases. Respir Med. 2016; 112:1–9. PMID: 26880379.
Article21. Sharif K, Watad A, Bragazzi NL, Adawi M, Amital H, Shoenfeld Y. Coffee and autoimmunity: more than a mere hot beverage! Autoimmun Rev. 2017; 16:712–721. PMID: 28479483.
Article22. Peng HJ, Chang ZN, Han SH, Won MH, Huang BT. Chemical denaturation of ovalbumin abrogates the induction of oral tolerance of specific IgG antibody and DTH responses in mice. Scand J Immunol. 1995; 42:297–304. PMID: 7544908.
Article23. Khan DA, Oppenheimer JJ, Lee GB, Fineman SM. Asthma. Ann Allergy Asthma Immunol. 2019; 123:416–417. PMID: 31351982.
Article24. Kelada SN. Plethysmography phenotype QTL in mice before and after allergen sensitization and challenge. G3 (Bethesda). 2016; 6:2857–2865. PMID: 27449512.
Article25. Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015; 16:45–56. PMID: 25521684.
Article26. Goto M, Yamaki K, Shinmoto H, Takano-Ishikawa Y. Continuous orally administered coffee enhanced the antigen-specific Th1 response and reduced allergic development in a TCR-transgenic mice model. Biosci Biotechnol Biochem. 2009; 73:2439–2444. PMID: 19897909.
Article27. Lajunen K, Kalliola S, Kotaniemi-Syrjänen A, Sarna S, Malmberg LP, Pelkonen AS, Mäkelä MJ. Abnormal lung function at preschool age asthma in adolescence? Ann Allergy Asthma Immunol. 2018; 120:520–526. PMID: 29522812.
Article28. Brigham EP, West NE. Diagnosis of asthma: diagnostic testing. Int Forum Allergy Rhinol. 2015; 5(Suppl 1):S27–S30. PMID: 26335833.
Article29. Forte GC, da Silva DT, Hennemann ML, Sarmento RA, Almeida JC, de Tarso Roth Dalcin P. Diet effects in the asthma treatment: a systematic review. Crit Rev Food Sci Nutr. 2018; 58:1878–1887. PMID: 28362110.
Article30. Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006; 111:877–892. PMID: 16540173.
Article31. Pohanka M. Caffeine downregulates antibody production in a mouse model. J Appl Biomed. 2014; 13:1–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dietary habits score, nutrients intake and dietary quality related to coffee consumption of college students in Incheon
- Coffee intake can promote activity of antioxidant enzymes with increasing MDA level and decreasing HDL-cholesterol in physically trained rats
- Coffee Intake and Risk of Hypertension: A Meta-Analysis of Cohort Studies
- Association with obesity and abdominal obesity according to the kind and amount of coffee intake in Korean adults: 2013 ~ 2016 Korea National Health and Nutrition Examination Survey
- A Study Evaluating Nutrient Intake and Diet Quality in Female College Students According to Coffee Consumption