Ann Surg Treat Res.
2023 Jul;105(1):31-36. 10.4174/astr.2023.105.1.31.
Can chemotherapy be omitted for patients with N0 or N1 endocrine-sensitive breast cancer treated with gonadotropin-releasing hormone agonist and tamoxifen?
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 2Division of Fusion Radiology Research, Korea Institute of Radiological and Medical Sciences, Seoul, Korea
- 3Department of Surgery, Konkuk University Medical Center, Seoul, Korea
- KMID: 2544610
- DOI: http://doi.org/10.4174/astr.2023.105.1.31
Abstract
- Purpose
Whether administering chemotherapy followed by tamoxifen plus a gonadotropin-releasing hormone (GnRH) agonist to treat patients with lower-risk hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer provides a greater benefit than administering tamoxifen plus GnRH agonist alone remains unclear. This study aimed to compare the outcomes of propensity score-matched (PSM) patients who underwent these 2 types of treatment plans.
Methods
This retrospective study included patients treated at our institution between 2009 and 2019. Eligible patients had HR-positive, HER2-negative, invasive breast cancer who had undergone surgery. There were 579 patients with HR-positive, HER2-negative breast cancer who were treated with a GnRH agonist and tamoxifen; patients with pathologic N2 and those who received neoadjuvant chemotherapy were excluded. After 1:1 PSM of patients who underwent GnRH agonist treatment and tamoxifen with versus without chemotherapy, 122 patients from these 2 groups were analyzed. Survival rates were calculated using the Kaplan-Meier method and compared via the log-rank test.
Results
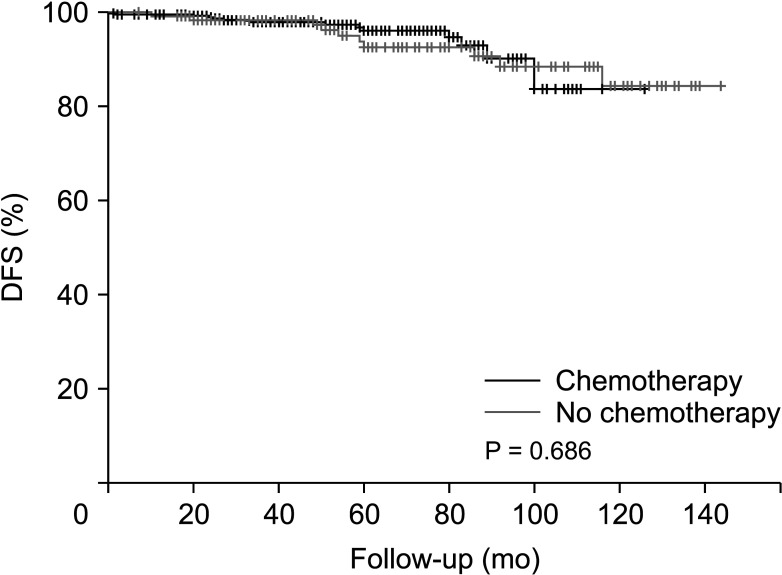
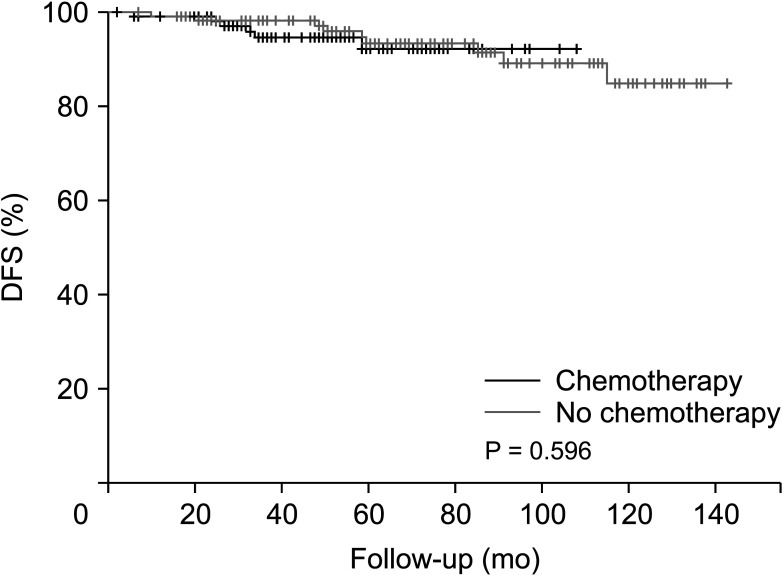
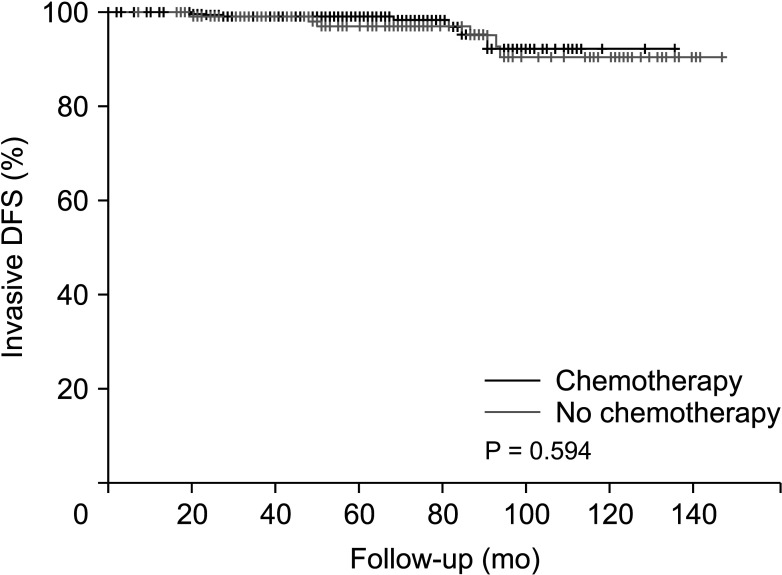
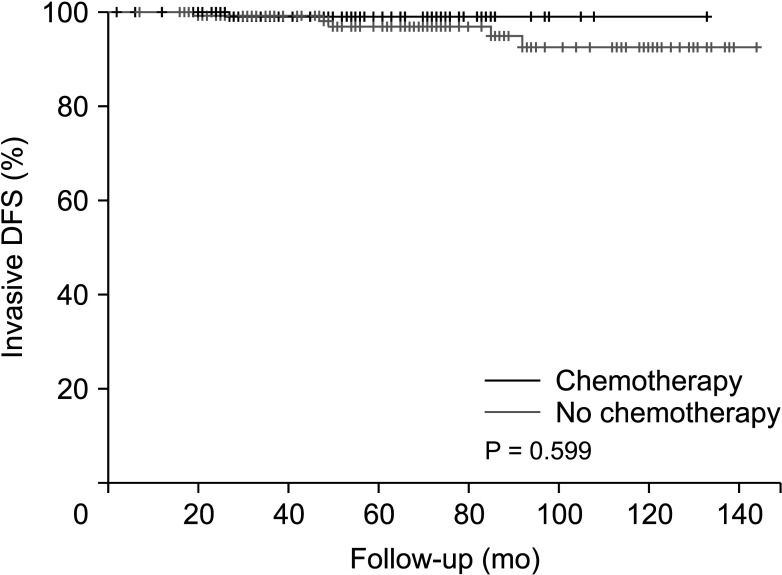
After PSM, there were no significant differences in several baseline characteristics between the 2 groups. After a median follow-up of 62.8 months, the patients in both groups demonstrated similar outcomes with no significant difference in disease-free survival (P = 0.596).
Conclusion
Patients derived no significant survival benefit from undergoing a chemotherapy regimen before receiving tamoxifen and GnRH agonist therapy compared to forgoing such chemotherapy.
Keyword
Figure
Reference
-
1. Salgado R, Peg V, Rüschoff J, Vincent-Salomon A, Castellano I, Perner S, et al. Gene expression signatures for tailoring adjuvant chemotherapy of luminal breast cancer: the pathologists’ perspective. Ann Oncol. 2021; 32:1316–1321. PMID: 34461263.2. Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009; 360:790–800. PMID: 19228622.3. Azim HA Jr, Michiels S, Zagouri F, Delaloge S, Filipits M, Namer M, et al. Utility of prognostic genomic tests in breast cancer practice: the IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013; 24:647–654. PMID: 23337633.4. Vieira AF, Schmitt F. An update on breast cancer multigene prognostic tests-emergent clinical biomarkers. Front Med (Lausanne). 2018; 5:248. PMID: 30234119.5. Winchester DP. Breast cancer in young women. Surg Clin North Am. 1996; 76:279–287. PMID: 8610264.6. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea. A report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–2368. PMID: 17515570.7. Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thürlimann B, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000; 355:1869–1874. PMID: 10866443.8. Kangleon-Tan HL, Sim J, You JY, Lee ES, Lee H, Yang SM, et al. Omission of chemotherapy for hormone receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: patterns of treatment and outcomes from the Korean Breast Cancer Society Registry. Ann Surg Treat Res. 2022; 103:313–322. PMID: 36601341.9. Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010; 362:2053–2065. PMID: 20519679.10. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018; 379:111–121. PMID: 29860917.11. Sparano JA, Gray RJ, Ravdin PM, Makower DF, Pritchard KI, Albain KS, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019; 380:2395–2405. PMID: 31157962.12. Gonzalez-Angulo AM, Barlow WE, Gralow J, Meric-Bernstam F, Hayes DF, Moinpour C, et al. SWOG S1007: A phase III, randomized clinical trial of standard adjuvant endocrine therapy with or without chemotherapy in patients with one to three positive nodes, hormone receptor (HR)-positive, and HER2-negative breast cancer with recurrence score (RS) of 25 or less. J Clin Oncol. 2011; 29(15 Suppl):TPS104.13. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021; 385:2336–2347. PMID: 34914339.14. Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020; 18:452–478. PMID: 32259783.15. Chung CC, Fleming R, Jamieson ME, Yates RW, Coutts JR. Randomized comparison of ovulation induction with and without intrauterine insemination in the treatment of unexplained infertility. Hum Reprod. 1995; 10:3139–3141. PMID: 8822431.16. Montemurro F, Perrone F, Geuna E. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015; 372:1672–1673.17. Sa-Nguanraksa D, Krisorakun T, Pongthong W, O-Charoenrat P. Survival outcome of combined GnRH agonist and tamoxifen is comparable to that of sequential adriamycin and cyclophosphamide chemotherapy plus tamoxifen in premenopausal patients with early breast cancer. Mol Clin Oncol. 2019; 11:517–522. PMID: 31620283.18. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018; 379:122–137. PMID: 29863451.19. Nurgali K, Jagoe RT, Abalo R. Editorial: adverse effects of cancer chemotherapy. Anything new to improve tolerance and reduce sequelae? Front Pharmacol. 2018; 9:245. PMID: 29623040.20. Azim HA Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011; 22:1939–1947. PMID: 21289366.21. Al-Mahayri ZN, Patrinos GP, Ali BR. Toxicity and pharmacogenomic biomarkers in breast cancer chemotherapy. Front Pharmacol. 2020; 11:445. PMID: 32351390.22. Park MJ, Park YH, Ahn HJ, Choi W, Paik KH, Kim JM, et al. Secondary hematological malignancies after breast cancer chemotherapy. Leuk Lymphoma. 2005; 46:1183–1188. PMID: 16085560.23. Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008; 17:317–328. PMID: 17721909.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endocrine Therapy for Breast Cancer
- In vitro respones of gynecological cancer cell lines to the GnRH agonist, medroxyprogesterone acetate and tamoxifen
- Role of Ovarian Function Suppression in Premenopausal Women with Early Breast Cancer
- Survival Outcome of Combined GnRH Agonist and Tamoxifen Is Comparable to That of Sequential Adriamycin and Cyclophosphamide Chemotherapy Plus Tamoxifen in Premenopausal Patients with Lymph-Node–Negative, Hormone-Responsive, HER2-Negative, T1-T2 Breast Cancer
- Efficacy of Combined Aromatase Inhibitor and Luteinizing Hormone-Releasing Hormone Agonist in Premenopausal Metastatic Breast Cancer