Blood Res.
2023 Jun;58(2):83-90. 10.5045/br.2023.2023005.
Advantage of achieving deep response following frontline daratumumab-VTd compared to VRd in transplant-eligible multiple myeloma: multicenter study
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Hematology, Seoul St. Mary’s Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Hematology, Yeoido St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Hematology, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Department of Hematology, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- KMID: 2543948
- DOI: http://doi.org/10.5045/br.2023.2023005
Abstract
- Background
The goal of induction therapy for multiple myeloma (MM) is to achieve adequate disease control. Current guidelines favor triplet (bortezomib-lenalidomide-dexamethasone; VRd) or quadruplet regimens (daratumumab, bortezomib-thalidomide-dexamethasone; D-VTd). In the absence of a direct comparison between two treatment regimens, we conducted this study to compare the outcomes and safety of VRd and D-VTd.
Methods
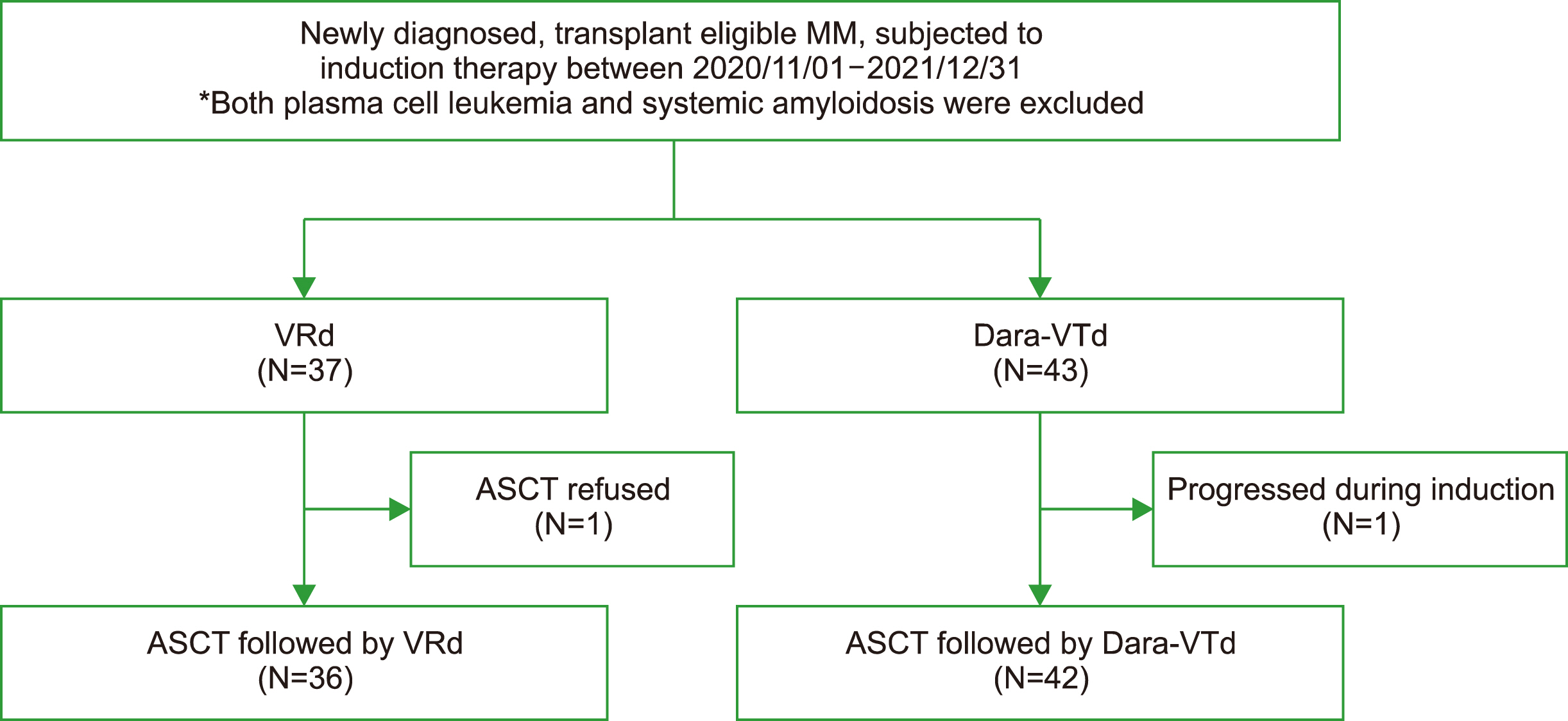
Newly diagnosed MM patients aged >18 years who underwent induction therapy followed by autologous stem cell transplantation (ASCT) between November 2020 and December 2021 were identified. Finally, patients with VRd (N=37) and those with D-VTd (N=43) were enrolled.
Results
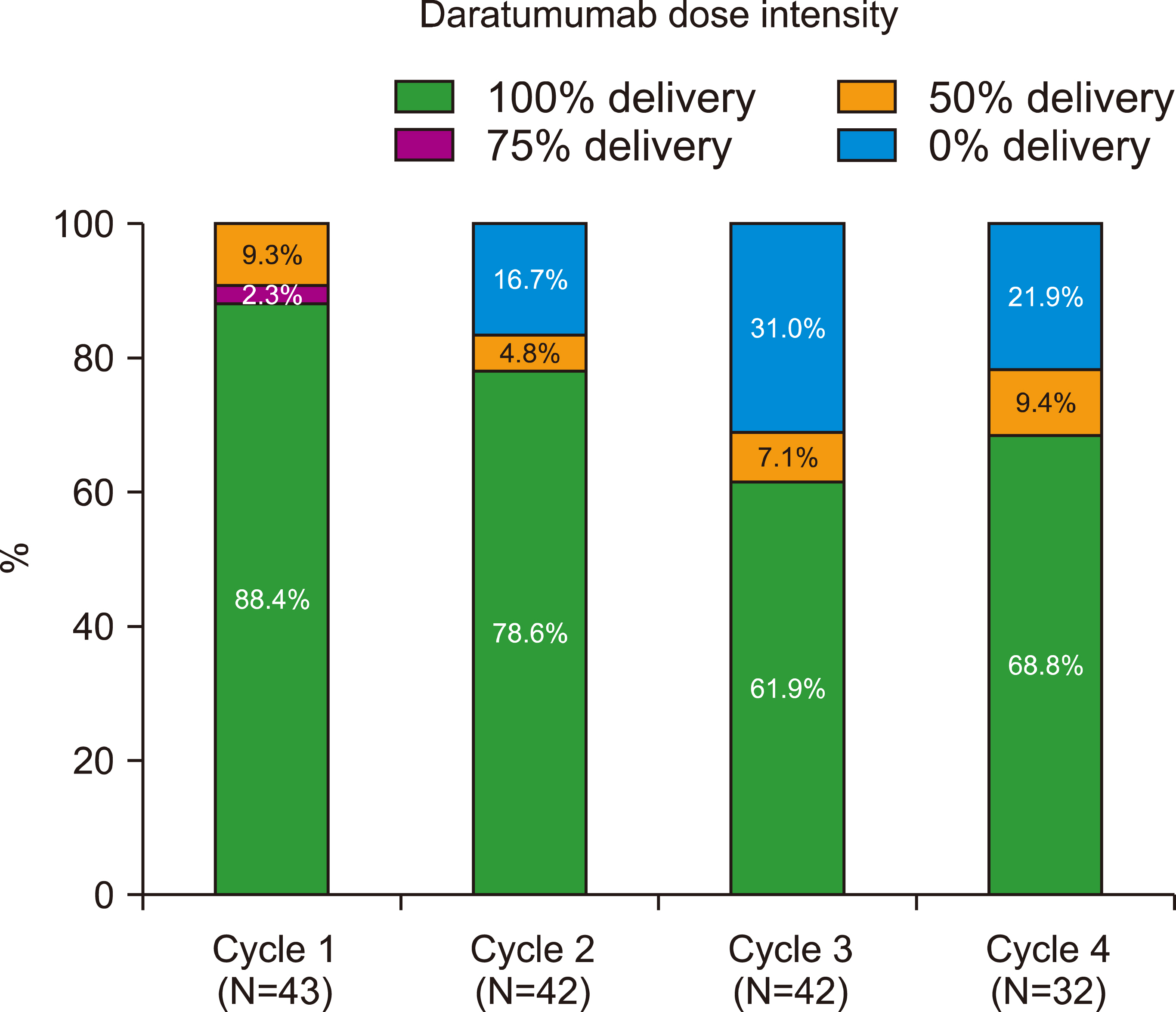
After induction, 10.8% of the VRd group showed stringent complete remission (sCR), 21.6% showed complete response (CR), 35.1% showed very good partial response (VGPR), and 32.4% showed partial response (PR). Of the D-VTd group, 9.3% showed sCR, 34.9% CR, 48.8% VGPR, and 4.2% PR (VGPR or better: 67.6% in VRd vs. 93% in D-VTd, P =0.004). After ASCT, 68.6% of the VRd group showed CR or sCR, while 90.5% of the D-VTd group showed CR or sCR (P=0.016). VRd was associated with an increased incidence of skin rash (P=0.044). Other than rashes, there were no significant differences in terms of adverse events between the two groups.
Conclusion
Our study supports the use of a front-line quadruplet induction regimen containing a CD38 monoclonal antibody for transplant-eligible patients with newly diagnosed MM.
Figure
Reference
-
1. Dimopoulos MA, Moreau P, Terpos E, et al. 2021; Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Hemasphere. 5:e528. DOI: 10.1097/HS9.0000000000000567. PMID: 33824948. PMCID: PMC8016603. PMID: d409ef3dc344467d8367eb32dafc60c9.2. Callander NS, Baljevic M, Adekola K, et al. 2022; NCCN GuidelinesⓇ Insights: multiple myeloma, version 3.2022. J Natl Compr Canc Netw. 20:8–19. DOI: 10.6004/jnccn.2022.0002. PMID: 34991075.3. Rosiñol L, Oriol A, Teruel AI, et al. 2012; Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretrans-plantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 120:1589–96. DOI: 10.1182/blood-2012-02-408922. PMID: 22791289.
Article4. Moreau P, Hulin C, Macro M, et al. 2016; VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 127:2569–74. DOI: 10.1182/blood-2016-01-693580. PMID: 27002117.5. Attal M, Lauwers-Cances V, Hulin C, et al. 2017; Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 376:1311–20. DOI: 10.1056/NEJMoa1611750. PMID: 28379796. PMCID: PMC6201242.
Article6. Rosiñol L, Oriol A, Rios R, et al. 2019; Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 134:1337–45. DOI: 10.1182/blood.2019000241. PMID: 31484647. PMCID: PMC6888142.
Article7. Bazarbachi AH, Al Hamed R, Malard F, Bazarbachi A, Harousseau JL, Mohty M. 2022; Induction therapy prior to autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma: an update. Blood Cancer J. 12:47. DOI: 10.1038/s41408-022-00645-1. PMID: 35347107. PMCID: PMC8960754. PMID: a9fde50dfe9f4bd3bae2643256c97f11.
Article8. Moreau P, Attal M, Hulin C, et al. 2019; Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 394:29–38. DOI: 10.1016/S0140-6736(19)31240-1. PMID: 31171419.9. Moreau P, Hulin C, Perrot A, et al. 2021; Maintenance with daratumumab or observation following treatment with bortezomib, thalidomide, and dexamethasone with or without daratumumab and autologous stem-cell transplant in patients with newly diagnosed multiple myeloma (CASSIOPEIA): an open-label, randomised, phase 3 trial. Lancet Oncol. 22:1378–90. DOI: 10.1016/S1470-2045(21)00428-9. PMID: 34529931.
Article10. Voorhees PM, Kaufman JL, Laubach J, et al. 2020; Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 136:936–45. DOI: 10.1182/blood.2020005288. PMID: 32325490. PMCID: PMC7441167.
Article11. Moreau P, Hebraud B, Facon T, et al. 2021; Front-line daratumumab-VTd versus standard-of-care in ASCT-eligible multiple myeloma: matching-adjusted indirect comparison. Immunotherapy. 13:143–54. DOI: 10.2217/imt-2020-0266. PMID: 33228440.
Article12. Kumar S, Paiva B, Anderson KC, et al. 2016; International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17:e328–46. DOI: 10.1016/s1470-2045(16)30206-6.13. Blanes M, Lahuerta JJ, González JD, et al. 2013; Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 19:69–74. DOI: 10.1016/j.bbmt.2012.08.009. PMID: 22897964.
Article14. Moreau P, Facon T, Attal M, et al. 2002; Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 99:731–5. DOI: 10.1182/blood.V99.3.731. PMID: 11806971.
Article15. Blum A, Haussmann K, Streitz M, et al. 2019; Standardized assay for assessment of minimal residual disease in blood, bone marrow and apheresis from patients with plasma cell myeloma. Sci Rep. 9:2922. DOI: 10.1038/s41598-019-39631-2. PMID: 30814612. PMCID: PMC6393516.
Article16. Stetler-Stevenson M, Paiva B, Stoolman L, et al. 2016; Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytometry B Clin Cytom. 90:26–30. DOI: 10.1002/cyto.b.21249. PMID: 25907102. PMCID: PMC7511978.
Article17. Arroz M, Came N, Lin P, et al. 2016; Consensus guidelines on plasma cell myeloma minimal residual disease analysis and reporting. Cytometry B Clin Cytom. 90:31–9. DOI: 10.1002/cyto.b.21228. PMID: 25619868.
Article18. Dimopoulos MA, Terpos E, Chanan-Khan A, et al. 2010; Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 28:4976–84. DOI: 10.1200/JCO.2010.30.8791. PMID: 20956629.
Article19. Wanchoo R, Abudayyeh A, Doshi M, et al. 2017; Renal toxicities of novel agents used for treatment of multiple myeloma. Clin J Am Soc Nephrol. 12:176–89. DOI: 10.2215/CJN.06100616. PMID: 27654928. PMCID: PMC5220662.
Article20. Yadav P, Cook M, Cockwell P. 2016; Current trends of renal impairment in multiple myeloma. Kidney Dis (Basel). 1:241–57. DOI: 10.1159/000442511. PMID: 27536684. PMCID: PMC4934811.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Panagglutination on Antibody Identification in a Multiple Myeloma Patient Receiving Daratumumab
- Daratumumab in dialysis-dependent multiple myeloma
- Frontline therapy for newly diagnosed patients with multiple myeloma
- Optimal maintenance and consolidation therapy for multiple myeloma in actual clinical practice
- Predictive role of absolute lymphocyte count in daratumumab-treated patients with relapsed/ refractory multiple myeloma