Korean Circ J.
2023 Jun;53(6):387-403. 10.4070/kcj.2022.0301.
LOXL1-AS1 Aggravates Myocardial Ischemia/Reperfusion Injury Through the miR-761/PTEN Axis
- Affiliations
-
- 1Department of Cardiovascular Medicine, The First People’s Hospital of Chenzhou, Chenzhou, China
- 2Department of Intensive Care Unit (ICU), Hunan Chest Hospital, Changsha, China
- 3Translational Medicine Institute, The First People’s Hospital of Chenzhou, Chenzhou, China
- KMID: 2543051
- DOI: http://doi.org/10.4070/kcj.2022.0301
Abstract
- Background and Objectives
Myocardial ischemia and reperfusion injury (MIRI) has high morbidity and mortality worldwide. We aimed to explore the role of long noncoding RNA lysyl oxidase like 1 antisense RNA 1 (LOXL1-AS1) in cardiomyocyte pyroptosis.
Methods
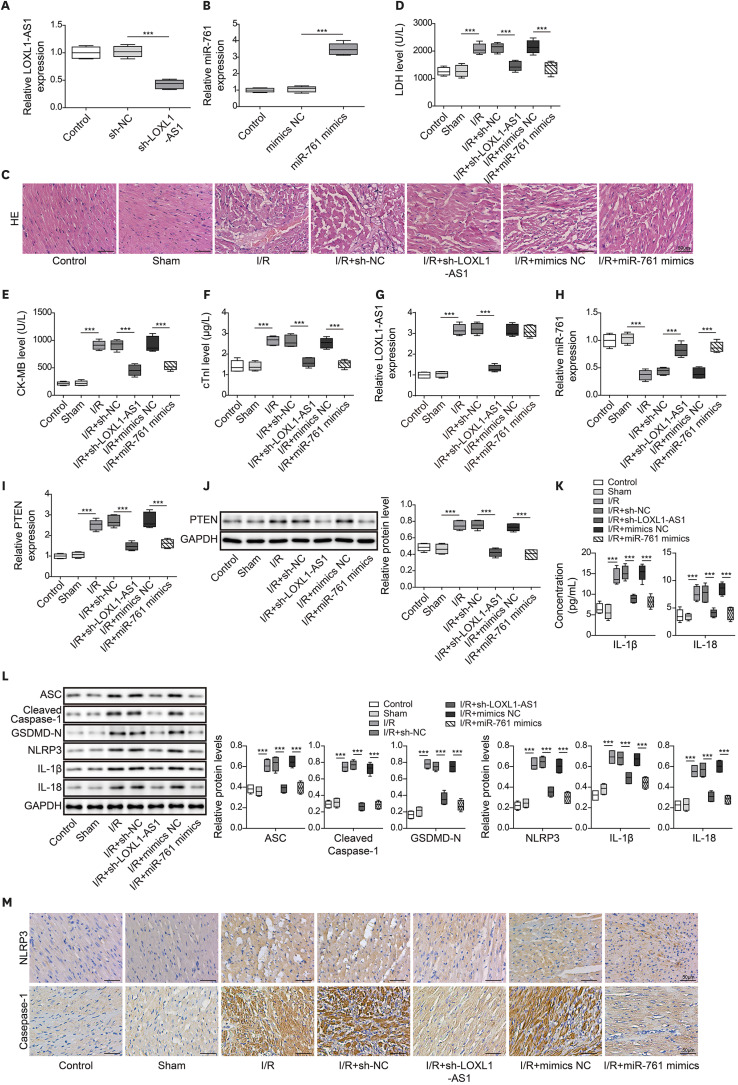
Hypoxia/reoxygenation (H/R) injury was constructed in human cardiomyocyte (HCM). The level of LOXL1-AS1, miR-761, phosphatase and tensin homolog (PTEN) and pyroptosis-related proteins was monitored by quantitative real-time polymerase chain reaction or western blot. Flow cytometry examined the pyroptosis level. Lactate dehydrogenase (LDH), creatine kinase-MB and cardiac troponin I levels were detected by test kits. Enzyme-linked immunosorbent assay measured the release of inflammatory cytokines. Dual-luciferase assay validated the binding relationship among LOXL1-AS1, miR-761, and PTEN. Finally, ischemia/reperfusion (I/R) animal model was constructed. Hematoxylin and eosin staining assessed morphological changes of myocardial tissue. NOD-like receptor pyrin domain-containing protein 3 (NLRP3) and casepase-1 expression was determined by immunohistochemistry.
Results
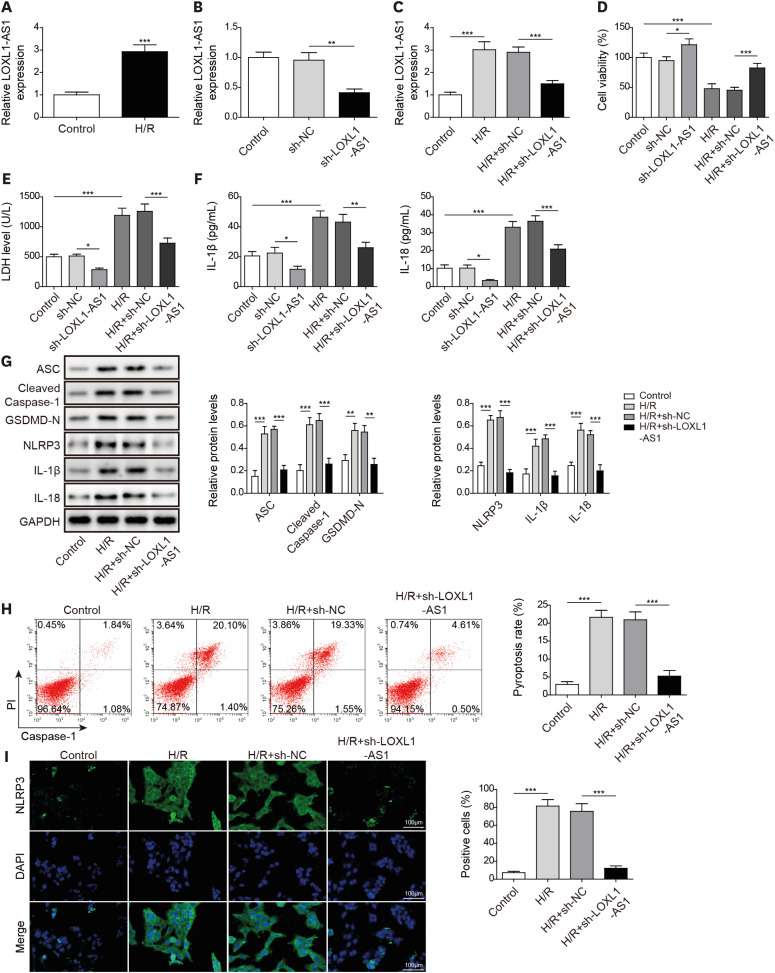
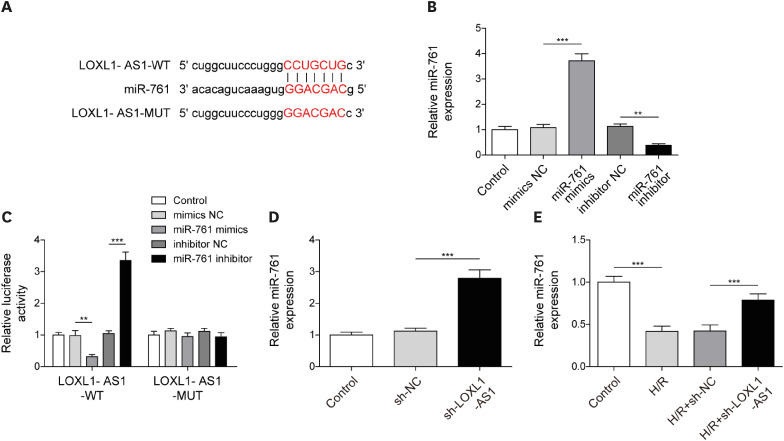
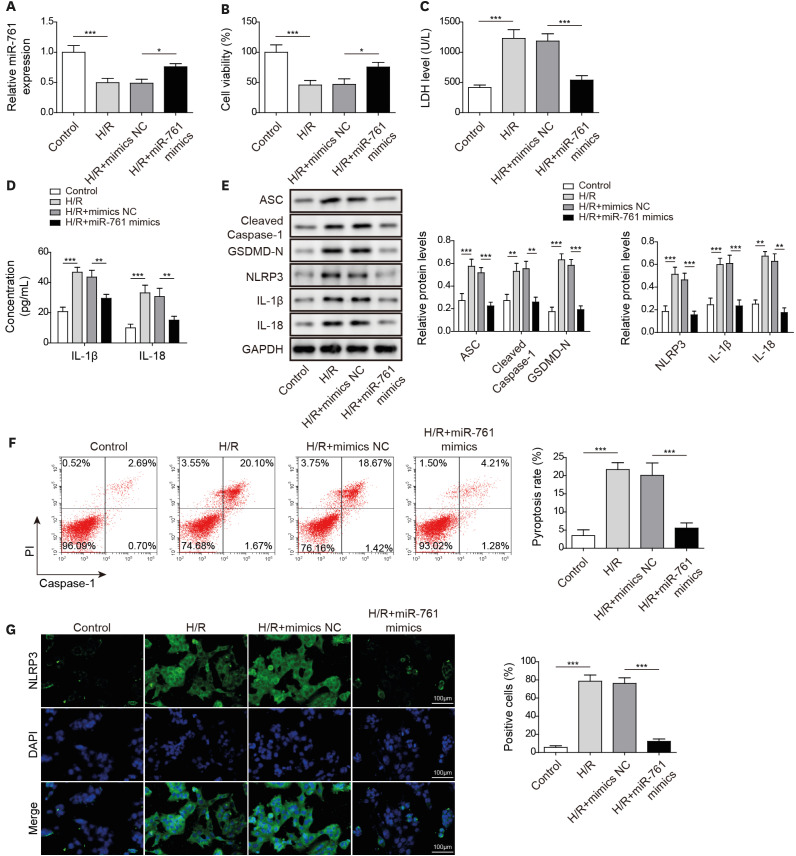
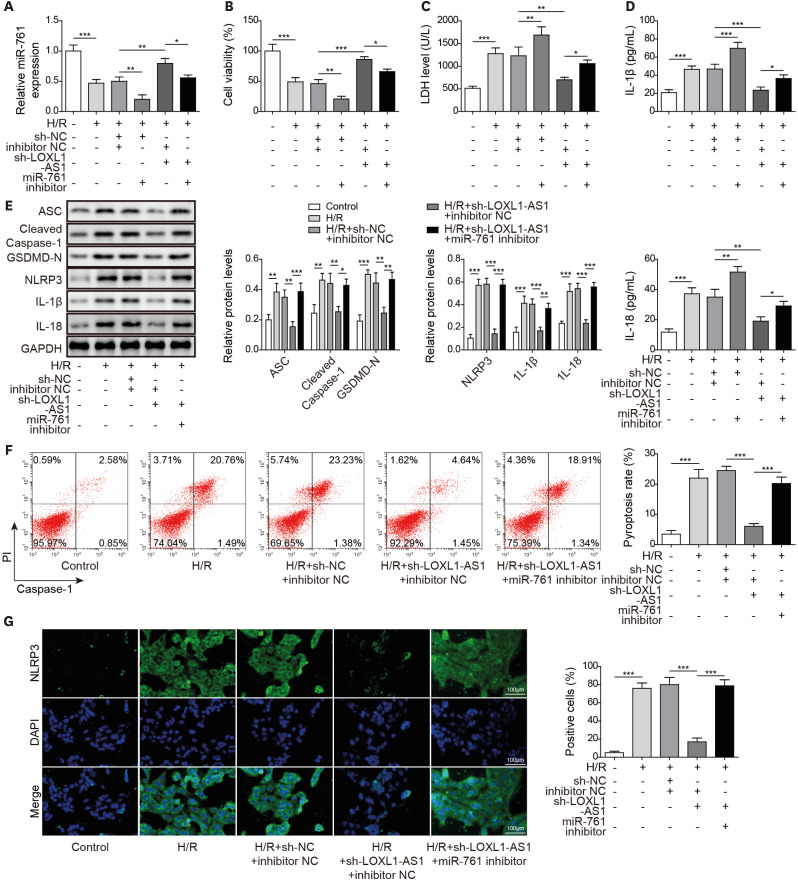
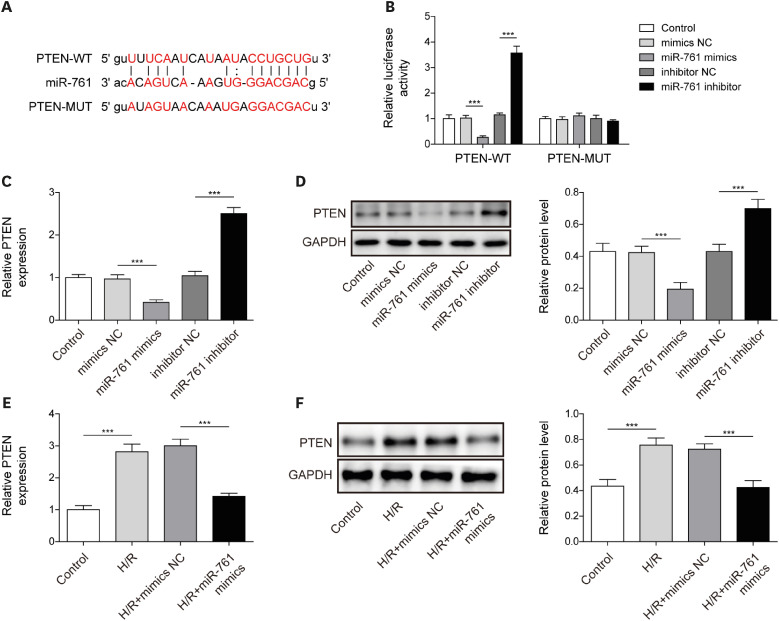
After H/R treatment, LOXL1-AS1 and PTEN were highly expressed but miR-761 level was suppressed. LOXL1-AS1 inhibition or miR-761 overexpression increased cell viability, blocked the release of LDH and inflammatory cytokines (interleukin [IL]-1β, IL-18), inhibited pyroptosis level, and downregulated pyroptosis-related proteins (ASC, cleaved caspase-1, gasdermin D-N, NLRP3, IL-1β, and IL-18) levels in HCMs. LOXL1-AS1 sponged miR-761 to upregulate PTEN. Knockdown of miR-761 reversed the effect of LOXL1-AS1 down regulation on H/R induced HCM pyroptosis. LOXL1-AS1 aggravated the MIRI by regulating miR-761/PTEN axis in vivo.
Conclusions
LOXL1-AS1 targeted miR-761 to regulate PTEN expression, then enhance cardiomyocyte pyroptosis, providing a new alternative target for the treatment of MIRI.
Keyword
Figure
Reference
-
1. Casin KM, Calvert JW. Dynamic regulation of cysteine oxidation and phosphorylation in myocardial ischemia-reperfusion injury. Cells. 2021; 10:2388. PMID: 34572037.2. Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018; 315:H1553–H1568. PMID: 30168729.3. Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009; 7:99–109. PMID: 19148178.4. Fang Y, Tian S, Pan Y, et al. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020; 121:109595. PMID: 31710896.5. Zhang J, Huang L, Shi X, et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY). 2020; 12:24270–24287. PMID: 33232283.6. Ye B, Chen X, Dai S, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019; 13:975–990.7. Joshi M, Rajender S. Long non-coding RNAs (lncRNAs) in spermatogenesis and male infertility. Reprod Biol Endocrinol. 2020; 18:103. PMID: 33126901.8. Sun Q, Li J, Li F, et al. LncRNA LOXL1-AS1 facilitates the tumorigenesis and stemness of gastric carcinoma via regulation of miR-708-5p/USF1 pathway. Cell Prolif. 2019; 52:e12687. PMID: 31468594.9. Song B, Dang H, Dong R. Differential expression of LOXL1-AS1 in coronary heart disease and its regulatory mechanism in ox-LDL-induced human coronary artery endothelial cell pyroptosis. Cardiovasc Drugs Ther. 2023; 37:75–87. PMID: 34633594.10. Bushati N, Cohen SM. MicroRNA functions. Annu Rev Cell Dev Biol. 2007; 23:175–205. PMID: 17506695.11. Ding S, Liu D, Wang L, Wang G, Zhu Y. Inhibiting microRNA-29a protects myocardial ischemia-reperfusion injury by targeting sirt1 and suppressing oxidative stress and NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther. 2020; 372:128–135. PMID: 31481517.12. Zhou Y, Li KS, Liu L, Li SL. MicroRNA-132 promotes oxidative stress-induced pyroptosis by targeting sirtuin 1 in myocardial ischaemia-reperfusion injury. Int J Mol Med. 2020; 45:1942–1950. PMID: 32236570.13. Wang C, Yang W, Liang X, et al. MicroRNA-761 modulates foam cell formation and inflammation through autophagy in the progression of atherosclerosis. Mol Cell Biochem. 2020; 474:135–146. PMID: 32772311.14. Xiang X, Zheng L, Li X. Silencing of long noncoding RNA zinc finger antisense 1 protects against hypoxia/reoxygenation-induced injury in HL-1 cells through targeting the miR-761/cell death inducing p53 target 1 axis. J Cardiovasc Pharmacol. 2020; 76:564–573. PMID: 32833901.15. Long B, Wang K, Li N, et al. MiR-761 regulates the mitochondrial network by targeting mitochondrial fission factor. Free Radic Biol Med. 2013; 65:371–379. PMID: 23867156.16. Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012; 16:123–132. PMID: 22368166.17. Bian Y, Li X, Pang P, et al. Kanglexin, a novel anthraquinone compound, protects against myocardial ischemic injury in mice by suppressing NLRP3 and pyroptosis. Acta Pharmacol Sin. 2020; 41:319–326. PMID: 31645662.18. Liang YP, Liu Q, Xu GH, et al. The lncRNA ROR/miR-124-3p/TRAF6 axis regulated the ischaemia reperfusion injury-induced inflammatory response in human cardiac myocytes. J Bioenerg Biomembr. 2019; 51:381–392. PMID: 31768721.19. Tan JK, Ma XF, Wang GN, Jiang CR, Gong HQ, Liu H. LncRNA MIAT knockdown alleviates oxygen-glucose deprivation-induced cardiomyocyte injury by regulating JAK2/STAT3 pathway via miR-181a-5p. J Cardiol. 2021; 78:586–597. PMID: 34489160.20. Sun J, Zhu YM, Liu Q, et al. LncRNA ROR modulates myocardial ischemia-reperfusion injury mediated by the miR-185-5p/CDK6 axis. Lab Invest. 2022; 102:505–514. PMID: 35066566.21. Xie Q, Li F, Shen K, Luo C, Song G. LOXL1-AS1/miR-515-5p/STAT3 positive feedback loop facilitates cell proliferation and migration in atherosclerosis. J Cardiovasc Pharmacol. 2020; 76:151–158. PMID: 32453072.22. Li S, Sun Y, Song M, et al. NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight. 2021; 6:e146852. PMID: 34877938.23. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015; 526:660–665. PMID: 26375003.24. Wang Y, Liu Y, Fei A, Yu Z. LncRNA XIST facilitates hypoxia-induced myocardial cell injury through targeting miR-191-5p/TRAF3 axis. Mol Cell Biochem. 2022; 477:1697–1707. PMID: 35257270.25. Mo L, Jiang HB, Tian GR, Lu GJ. The proliferation and migration of atherosclerosis-related HVSMCs were inhibited by downregulation of lncRNA XIST via regulation of the miR-761/BMP9 axis. Kaohsiung J Med Sci. 2022; 38:18–29. PMID: 34595819.26. Ruan H, Li J, Ren S, et al. Inducible and cardiac specific PTEN inactivation protects ischemia/reperfusion injury. J Mol Cell Cardiol. 2009; 46:193–200. PMID: 19038262.27. Li G, Yang J, Yang C, et al. PTENα regulates mitophagy and maintains mitochondrial quality control. Autophagy. 2018; 14:1742–1760. PMID: 29969932.28. Cui Q, Wang J, Liu X, Wang X, Su G. Knockout of PTEN improves cardiac function and inhibits NLRP3-mediated cardiomyocyte pyroptosis in rats with myocardial ischemia-reperfusion. Xibao Yu Fenzi Mianyixue Zazhi. 2020; 36:205–211. PMID: 32389167.29. Dai ZH, Jiang ZM, Tu H, et al. MiR-129 attenuates myocardial ischemia reperfusion injury by regulating the expression of PTEN in rats. BioMed Res Int. 2021; 2021:5535788. PMID: 34435045.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LOXL1-AS1/miR-761/PTEN as a Novel Signaling Pathway in Myocardial Ischemia and Reperfusion Injury (MIRI): Epigenetic Regulation by Long Non-Coding RNA (LncRNA) in MIRI
- CircZNF609 Aggravated Myocardial Ischemia Reperfusion Injury via Mediation of miR-214-3p/PTGS2 Axis
- LncRNA DLG1-AS1 Promotes Cancer Cell Proliferation in Triple Negative Breast Cancer by Downregulating miR-203
- MiR-484 Protects Rat Myocardial Cells from Ischemia-Reperfusion Injury by Inhibiting Caspase-3 and Caspase-9 during Apoptosis
- Long Noncoding RNA FBXL19-AS1-Mediated Ulcerative Colitis-Associated Intestinal Epithelial Barrier Defect