Korean Circ J.
2020 Mar;50(3):250-263. 10.4070/kcj.2019.0107.
MiR-484 Protects Rat Myocardial Cells from Ischemia-Reperfusion Injury by Inhibiting Caspase-3 and Caspase-9 during Apoptosis
- Affiliations
-
- 1Department of Internal Medicine, The Graduate School of Jinzhou Medical University, Jinzhou, China.
- 2Department of Cardiology, The Fourth People's Hospital of Shenyang, Shenyang, China. yinjun2019@163.com
- KMID: 2470910
- DOI: http://doi.org/10.4070/kcj.2019.0107
Abstract
- BACKGROUND AND OBJECTIVES
To reveal the detail mechanism of miR-484 on myocardial ischemia-reperfusion (MI/R) injury.
METHODS
Rats model of MI/R injury was established based on control (Con; sham operate) group, ischemia-reperfusion (I/R) group, miR-484 treatment (miR) group, and I/R-negative control (IR-C) group, followed by pathological and interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β expression evaluation. Then the myocardial apoptosis, as well as the expression of miR-484, caspase-3, and caspase-9 in myocardium were examined. Finally, the regulatory relation between miR-484 and SMAD family member 7 (SMAD7) was predicated, followed by verification analysis.
RESULTS
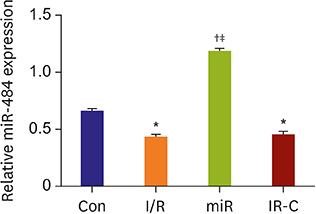
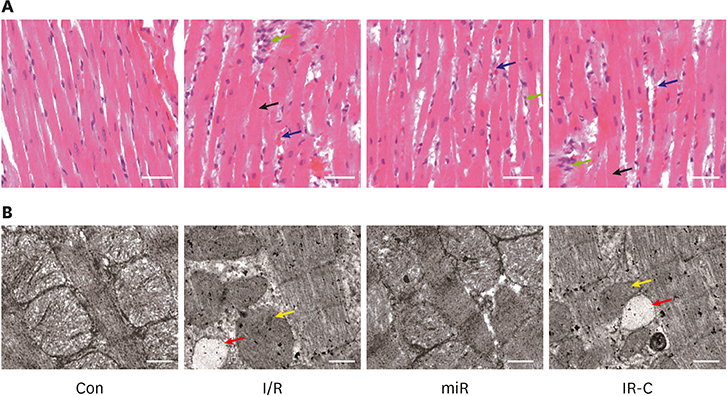
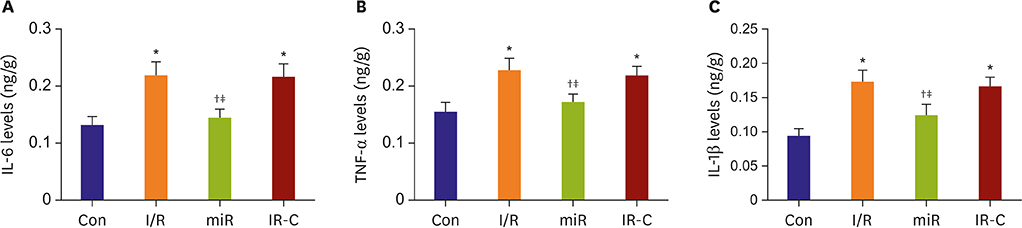
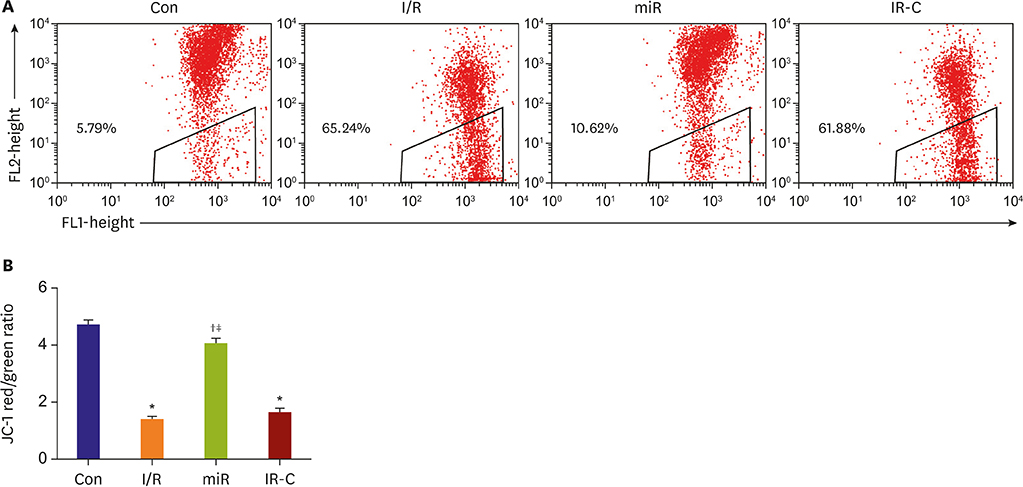
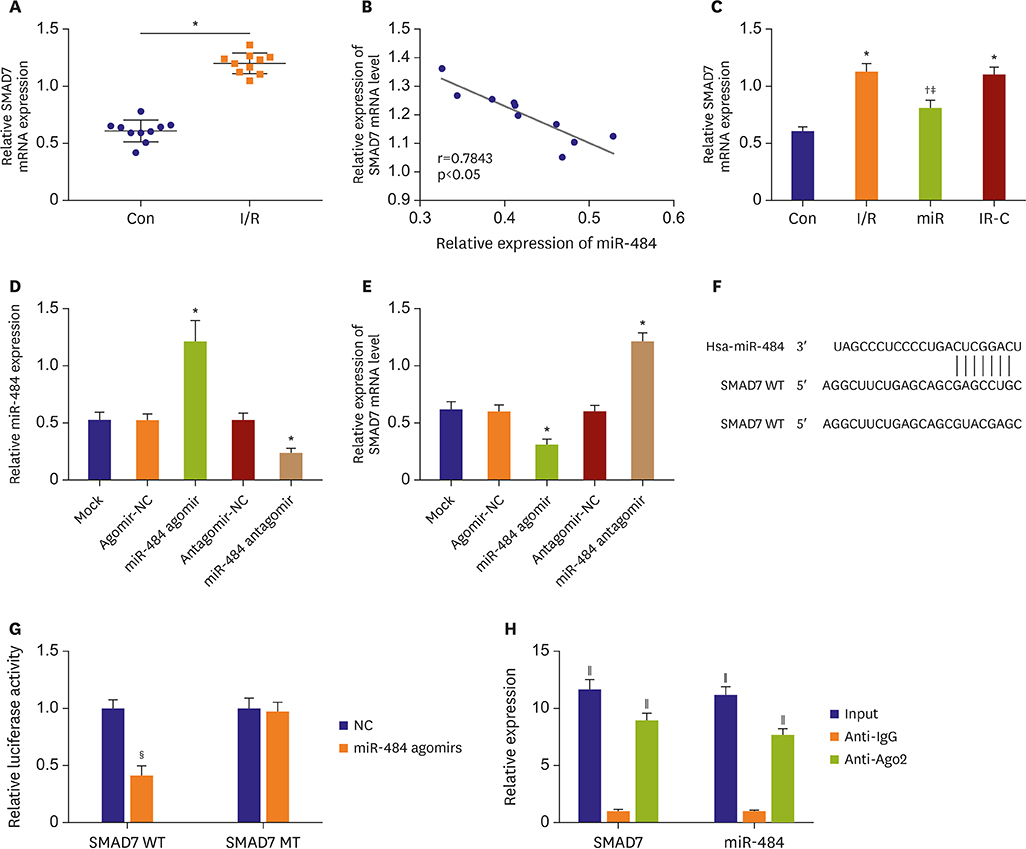
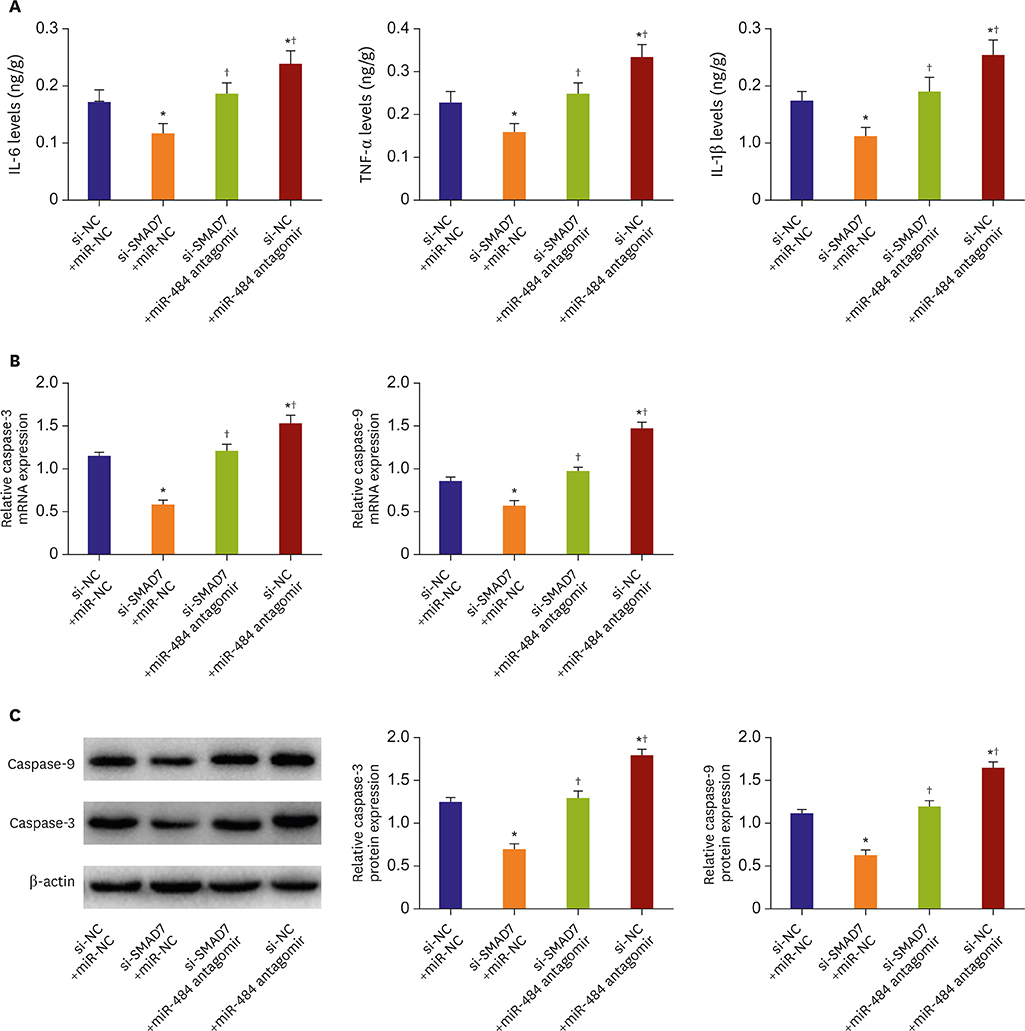
Compared with Con group, the expression of miR-484 in I/R and IR-C group was decreased. Compared with I/R and IR-C group, the expression of miR-484 was increased in miR group. Compared with Con group, the expression levels of IL-6, TNF-α, and IL-1β in cardiac myocytes of I/R group and IR-C group were increased. Compared with Con group, the apoptotic index, membrane potential of I/R, and the expression of caspase-3/9 were increased in IR-C group. Compared with the I/R and IR-C groups, the apoptotic index of myocardial cells in the ischemic region was decreased, the membrane potential was increased, and the expression of caspase-3/9 was decreased significantly in the miR group. SMAD7 was the target gene of miR-484.
CONCLUSIONS
MiR-484 protected myocardial cells from I/R injury by suppressing caspase-3 and caspase-9 expression during cardiomyocyte apoptosis. MiR-484 reduced the expression of IL-6, TNF-α, and IL-1β in MI/R. MiR-484 might alleviate the decreasing of mitochondrial membrane potential in MI/R cells.
MeSH Terms
Figure
Cited by 1 articles
-
A New Member of Myocardial Ischemia-Reperfusion (MI/R) Associated miRNAs, miR-484: Its Potential Cardiac Protection Role
Heeyoung Seok
Korean Circ J. 2020;50(3):264-266. doi: 10.4070/kcj.2019.0413.
Reference
-
1. Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013; 123:92–100.
Article2. Yuxian X, Jianfeng G, Ke L. Influence of electricity needle on nuclear transcription factor NF-κB and amino acid transmitters of aging rat's ischemia reperfusion brain tissue. Tradit Chin Med Orthop Med. 2010; 25:462. 465.3. Wang X, Zhang X, Ren XP, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010; 122:1308–1318.
Article4. Li J, Li L, Li X, Wu S. Long noncoding RNA LINC00339 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting miR-484. Biochem Biophys Res Commun. 2018; 503:3038–3043.
Article5. Loison F, Zhu H, Karatepe K, et al. Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J Clin Invest. 2014; 124:4445–4458.
Article6. Zhou X, Zhang J, Jia Q, et al. Reduction of miR-21 induces glioma cell apoptosis via activating caspase 9 and 3. Oncol Rep. 2010; 24:195–201.
Article7. Thukkani AK, Shoghi KI, Zhou D, et al. PET imaging of in vivo caspase-3/7 activity following myocardial ischemia-reperfusion injury with the radiolabeled isatin sulfonamide analogue [(18)F]WC-4-116. Am J Nucl Med Mol Imaging. 2016; 6:110–119.8. Sodhi RK, Singh M, Singh N, Jaggi AS. Protective effects of caspase-9 and poly(ADP-ribose) polymerase inhibitors on ischemia-reperfusion-induced myocardial injury. Arch Pharm Res. 2009; 32:1037–1043.
Article9. Kimuta M, Miura T, Okamura T, Iwamoto H, Iwatate M, Matsuzaki M. Effect of caspase-3 inhibitor on myocyte apoptosis and left ventricular remodeling in the ischemia-reperfused rat heart. J Card Fail. 1999; 5:48.
Article10. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408.11. Marrone AK, Beland FA, Pogribny IP. The role for microRNAs in drug toxicity and in safety assessment. Expert Opin Drug Metab Toxicol. 2015; 11:601–611.
Article12. Zhou Y, Chen Q, Lew KS, Richards AM, Wang P. Discovery of potential therapeutic miRNA targets in cardiac ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2016; 21:296–309.
Article13. Yu XY, Song YH, Geng YJ, et al. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008; 376:548–552.
Article14. Mutharasan RK, Nagpal V, Ichikawa Y, Ardehali H. microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. Am J Physiol Heart Circ Physiol. 2011; 301:H1519–30.
Article15. Aurora AB, Mahmoud AI, Luo X, et al. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012; 122:1222–1232.16. Yao Y, Sun F, Lei M. MiR-25 inhibits sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci Rep. 2018; 38:BSR20171511.
Article17. Ahmed FE, Jeffries CD, Vos PW, et al. Diagnostic microRNA markers for screening sporadic human colon cancer and active ulcerative colitis in stool and tissue. Cancer Genomics Proteomics. 2009; 6:281–295.18. Hu X, Ye F, Cao Z, et al. Abstract P4-07-11: dual characteristics of microRNA-484 modulated cytidine deaminase (CDA) axis in breast cancer: chemo-resistance and regulating cell proliferation. In : Thirty-Sixth Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2013 Dec 10–14; Tue, USA. San Antonio: American Association for Cancer Research;2013.19. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999; 6:99–104.
Article20. Blanc C, Deveraux QL, Krajewski S, et al. Caspase-3 is essential for procaspase-9 processing and cisplatin-induced apoptosis of MCF-7 breast cancer cells. Cancer Res. 2000; 60:4386–4390.21. Li T, Ding ZL, Zheng YL, Wang W. MiR-484 promotes non-small-cell lung cancer (NSCLC) progression through inhibiting Apaf-1 associated with the suppression of apoptosis. Biomed Pharmacother. 2017; 96:153–164.
Article22. Lunsford KE, Baird BJ, Sempowski GD, et al. Upregulation of IL-1β, IL-6, and CCL-2 by a novel mouse model of pancreatic ischemia-reperfusion injury. Transplantation. 2013; 95:1000–1007.
Article23. Yamada T, Murase N, Maeda T, et al. Protective effect of TNF-α and IL-1 β inhibitor FR167653 on ischemia-reperfusion injury in rat small intestinal transplantation. Transplant Proc. 1998; 30:2638.24. Kokkinopoulos I, Colman A, Hogg C, Heckenlively J, Jeffery G. Age-related retinal inflammation is reduced by 670 nm light via increased mitochondrial membrane potential. Neurobiol Aging. 2013; 34:602–609.
Article25. Ylitalo KV, Ala-Rämi A, Liimatta EV, Peuhkurinen KJ, Hassinen IE. Intracellular free calcium and mitochondrial membrane potential in ischemia/reperfusion and preconditioning. J Mol Cell Cardiol. 2000; 32:1223–1238.
Article26. Sun CK, Zhang XY, Sheard PW, Mabuchi A, Wheatley AM. Change in mitochondrial membrane potential is the key mechanism in early warm hepatic ischemia-reperfusion injury. Microvasc Res. 2005; 70:102–110.
Article27. Solhjoo S, O'Rourke B. Mitochondrial instability during regional ischemia-reperfusion underlies arrhythmias in monolayers of cardiomyocytes. J Mol Cell Cardiol. 2015; 78:90–99.
Article28. Chen Y, Li D. Smad7 and fibrotic disorders. Acta Universitatis Medicinalis Secondae Shanghai. 2005; 2:131–133.29. Zhang B, Zhou M, Li C, et al. MicroRNA-92a inhibition attenuates hypoxia/reoxygenation-induced myocardiocyte apoptosis by targeting Smad7. PLoS One. 2014; 9:e100298.
Article30. Yang Y, Ding S, Xu G, Chen F, Ding F. MicroRNA-15a inhibition protects against hypoxia/reoxygenation-induced apoptosis of cardiomyocytes by targeting mothers against decapentaplegic homolog 7. Mol Med Rep. 2017; 15:3699–3705.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of alpha-Lipoic Acid on Apoptotic Cell Death in Rat Hippocampus Following Transient Forebrain Ischemia-reperfusion Injury
- Apple pectin, a dietary fiber, ameliorates myocardial injury by inhibiting apoptosis in a rat model of ischemia/reperfusion
- Effect of Pretreatment of Mycophenolate Mofetil on Apoptotic Cell Death and Map Kinases Activation in Rat Kidneys with Ischemia/Reperfusion Injury
- Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice
- Supplementation with psyllium seed husk reduces myocardial damage in a rat model of ischemia/reperfusion