Cancer Res Treat.
2023 Apr;55(2):693-703. 10.4143/crt.2022.952.
Cyclophosphamide, Bortezomib, and Dexamethasone Consolidation in Patients with Multiple Myeloma after Stem Cell Transplantation: The KMM130 Study
- Affiliations
-
- 1Center for Hematologic Malignancies, National Cancer Center, Goyang, Korea
- 2Division of Hematology/Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 5Division of Hematology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 6Division of Hematology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Hematology-Oncology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 8Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 9Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea
- 10Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 11Targeted Therapy Branch, Research Institute, National Cancer Center, Goyang, Korea
- 12Department of Laboratory Medicine, Hospital, National Cancer Center, Goyang, Korea
- 13Department of Cancer Biomedical Science, National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea
- 14Department of Hematology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2541256
- DOI: http://doi.org/10.4143/crt.2022.952
Abstract
- Purpose
A three-drug combination of cyclophosphamide, bortezomib, and dexamethasone (CVD) shows significant efficacy and manageable toxicity as induction therapy in patients with multiple myeloma.
Materials and Methods
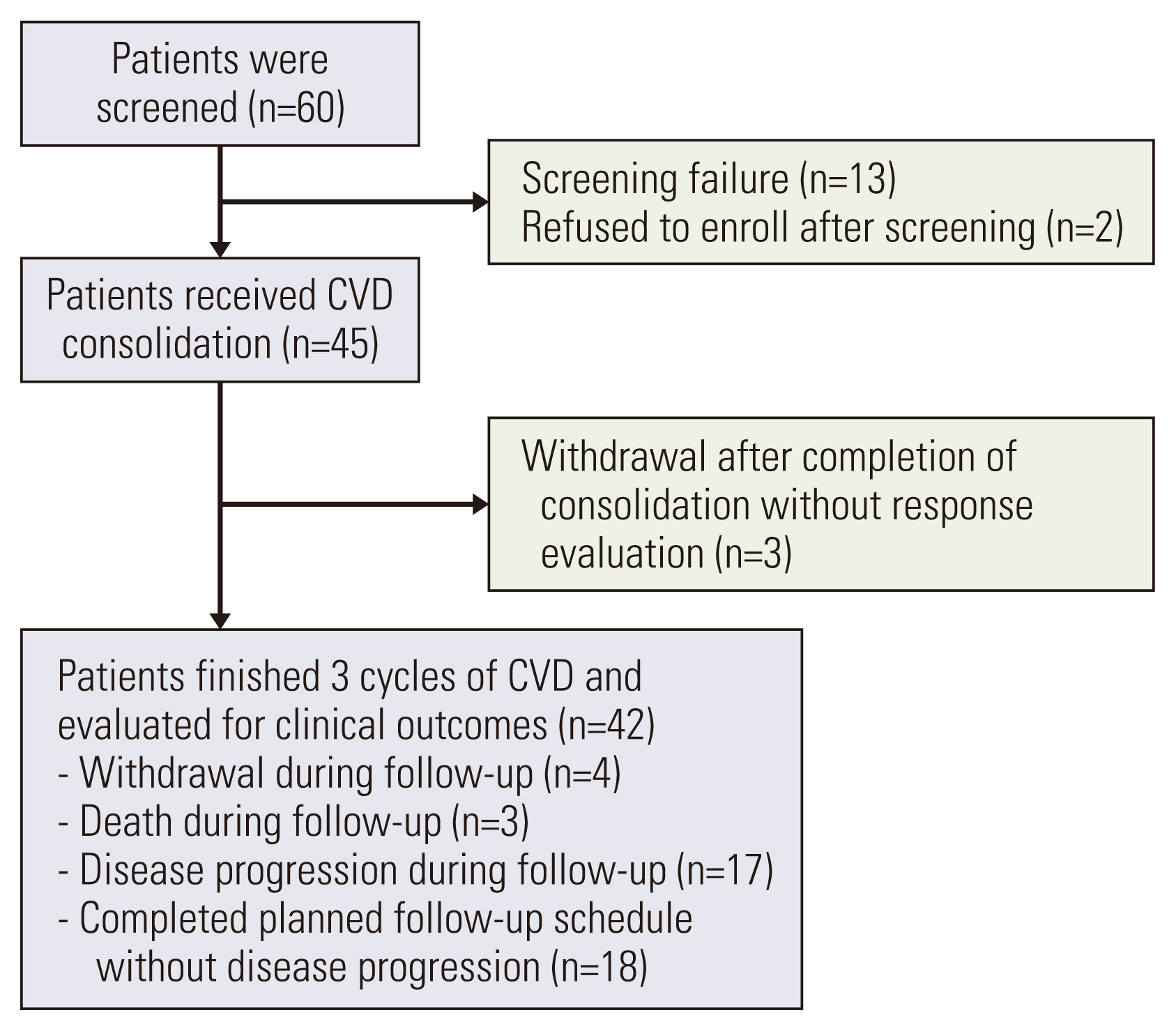
In this phase II study, we enrolled 45 patients who achieved a very good partial response (VGPR) or partial response (PR) after autologous stem cell transplantation (ASCT) and evaluated the efficacy and toxicity of CVD consolidation. CVD consolidation comprised three cycles of cyclophosphamide 300 mg/m2 orally on days 1, 8, and 15, and bortezomib 1.3 mg/m2 subcutaneously on days 1, 8, 15, and 22, along with dexamethasone 20 mg orally or intravenously on days 1 and 2, 8 and 9, 15 and 16, and 22 and 23.
Results
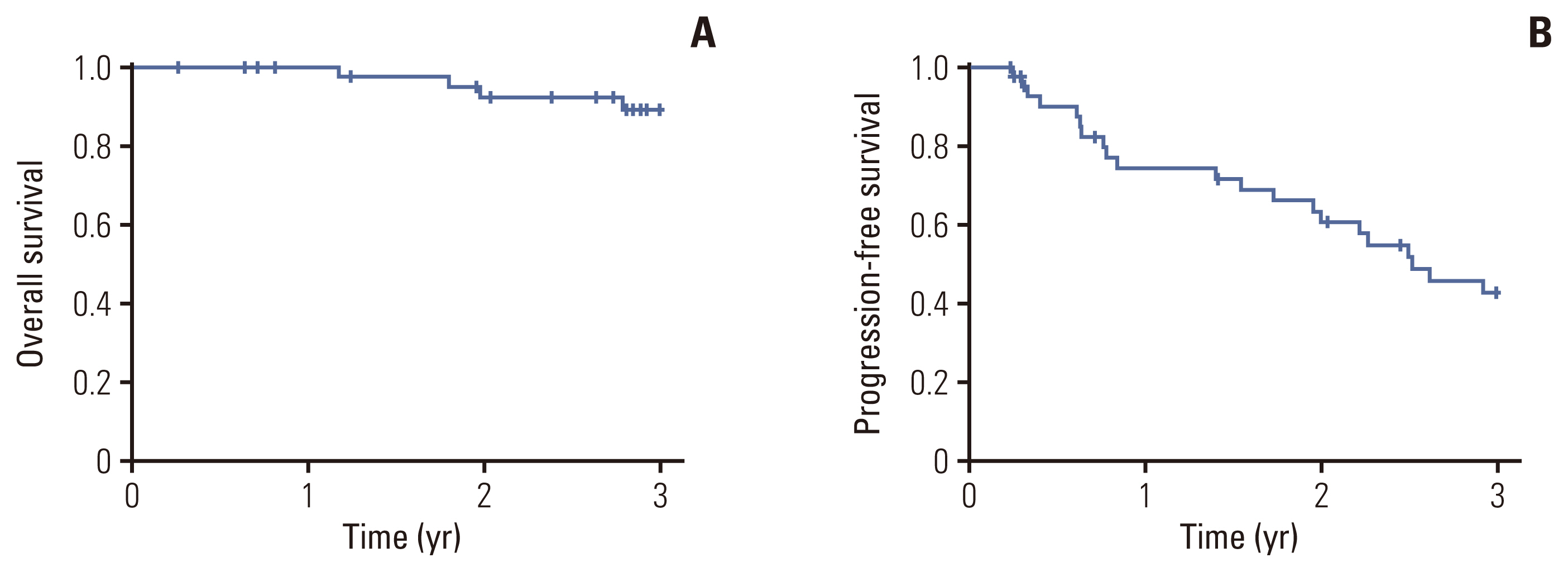
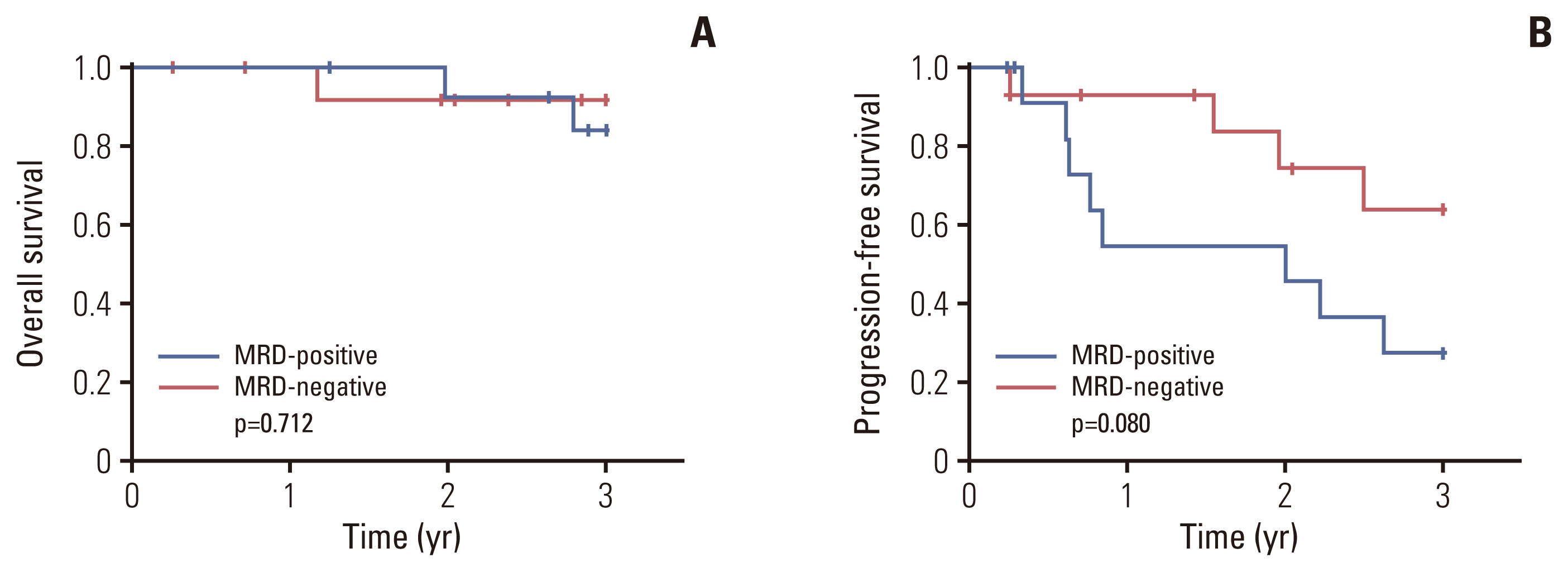
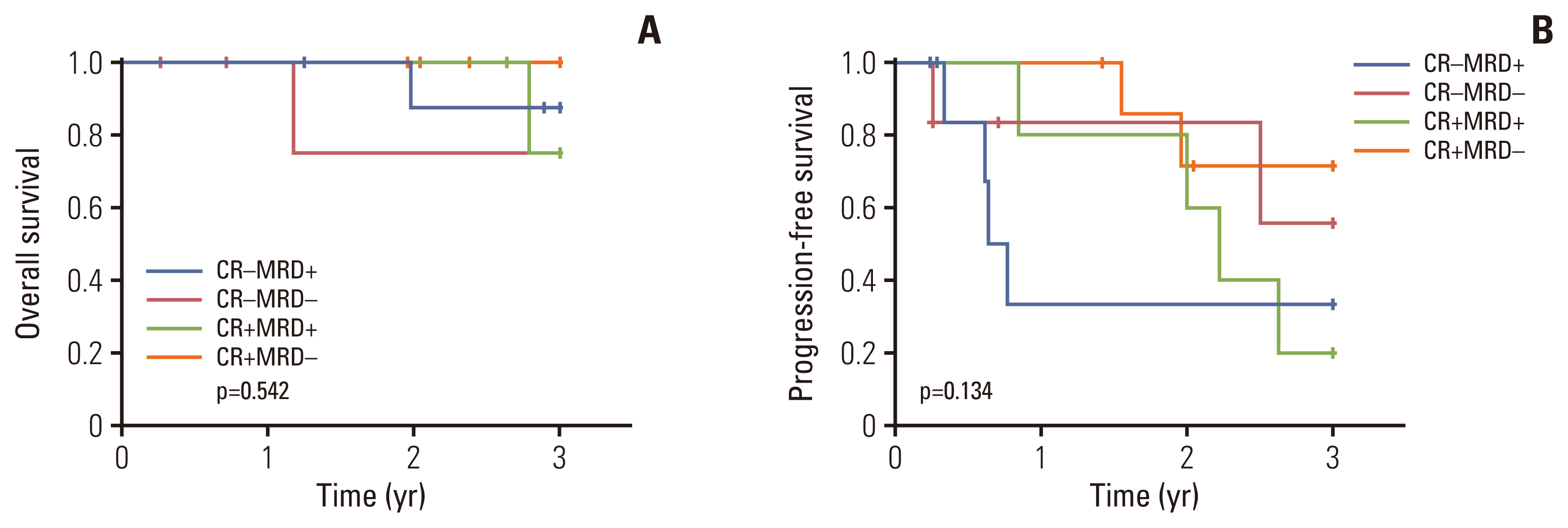
At enrollment, 39 patients (86.7%) showed VGPR, and nine (13.3%) presented with PR. Nineteen patients (45.2%) achieved a complete response or better as their best response after the end of consolidation. Overall, 22 of 42 patients (52.4%) experienced an improved response status with CVD consolidation. Three-year overall survival and progression-free survival rates were 89.0% and 42.7%, respectively. The most common non-hematologic toxicities were peripheral neuropathy and infection (20.5%), with no grade ≥ 3 neuropathy observed.
Conclusion
These results showed that CVD consolidation therapy improved the response with reasonable toxicity in patients with residual disease after ASCT. This trial was registered with the Clinical Research Information Service, Republic of Korea (KCT0001327).
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.2. Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996; 335:91–7.3. Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020; 136:936–45.4. Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019; 394:29–38.5. McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017; 35:3279–89.6. Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. J Clin Oncol. 2014; 32:2712–7.7. Jung J, Choi YS, Lee JH, Lee WS, Kim SH, Park Y, et al. Autologous stem cell transplantation in elderly patients with multiple myeloma in Korea: the KMM1807 study. Int J Hematol. 2020; 112:84–95.8. Rahman S, Rybicki L, Ky Hamilton B, Pohlman B, Jagadeesh D, Cober E, et al. Early infectious complications after autologous hematopoietic cell transplantation for multiple myeloma. Transpl Infect Dis. 2019; 21:e13114.9. Ladetto M, Pagliano G, Ferrero S, Cavallo F, Drandi D, Santo L, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol. 2010; 28:2077–84.10. Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. J Peripher Nerv Syst. 2008; 13:275–82.11. Kim YK, Sohn SK, Lee JH, Yang DH, Moon JH, Ahn JS, et al. Clinical efficacy of a bortezomib, cyclophosphamide, thalidomide, and dexamethasone (Vel-CTD) regimen in patients with relapsed or refractory multiple myeloma: a phase II study. Ann Hematol. 2010; 89:475–82.12. Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352:2487–98.13. Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro Coy N, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012; 97:442–50.14. Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009; 23:1337–41.15. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17:e328–46.16. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014; 25:2359–81.17. van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007; 92:1399–406.18. Mellqvist UH, Gimsing P, Hjertner O, Lenhoff S, Laane E, Remes K, et al. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial. Blood. 2013; 121:4647–54.19. Tacchetti P, Pantani L, Patriarca F, Petrucci MT, Zamagni E, Dozza L, et al. Bortezomib, thalidomide, and dexamethasone followed by double autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma (GIMEMA-MMY-3006): long-term follow-up analysis of a randomised phase 3, open-label study. Lancet Haematol. 2020; 7:e861–73.20. Rosinol L, Oriol A, Rios R, Sureda A, Blanchard MJ, Hernandez MT, et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood. 2019; 134:1337–45.21. Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020; 7:e456–68.22. Stadtmauer EA, Pasquini MC, Blackwell B, Hari P, Bashey A, Devine S, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: results of the BMT CTN 0702 trial. J Clin Oncol. 2019; 37:589–97.23. Zhang S, Kulkarni AA, Xu B, Chu H, Kourelis T, Go RS, et al. Bortezomib-based consolidation or maintenance therapy for multiple myeloma: a meta-analysis. Blood Cancer J. 2020; 10:33.24. Mills JR, Barnidge DR, Dispenzieri A, Murray DL. High sensitivity blood-based M-protein detection in sCR patients with multiple myeloma. Blood Cancer J. 2017; 7:e590.25. Mellors PW, Binder M, Buadi FK, Lacy MQ, Gertz MA, Dispenzieri A, et al. Time to plateau as a predictor of survival in newly diagnosed multiple myeloma. Am J Hematol. 2018; 93:889–94.26. Mariette X, Bergot C, Ravaud P, Roux C, Laval-Jeantet M, Brouet JC, et al. Evolution of bone densitometry in patients with myeloma treated with conventional or intensive therapy. Cancer. 1995; 76:1559–63.27. Sezer O. Myeloma bone disease: recent advances in biology, diagnosis, and treatment. Oncologist. 2009; 14:276–83.28. Terpos E, Dimopoulos MA, Sezer O, Roodman D, Abildgaard N, Vescio R, et al. The use of biochemical markers of bone remodeling in multiple myeloma: a report of the International Myeloma Working Group. Leukemia. 2010; 24:1700–12.29. Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006; 77:233–8.30. Tosi P, Zamagni E, Cellini C, Parente R, Cangini D, Tacchetti P, et al. First-line therapy with thalidomide, dexamethasone and zoledronic acid decreases bone resorption markers in patients with multiple myeloma. Eur J Haematol. 2006; 76:399–404.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal maintenance and consolidation therapy for multiple myeloma in actual clinical practice
- Efficacy and Safety of Melphalan, Cyclophosphamide and Dexamethasone (MCD) as a Salvage Treatment for Patients with Relapsed/Refractory Multiple Myeloma
- Diagnosis and therapy of multiple myeloma
- A Case of Drug-Induced Hepatitis due to Bortezomib in Multiple Myeloma
- A Case of Acute Pancreatitis Caused by Bortezomib in a Patient with Multiple Myeloma