Cancer Res Treat.
2023 Apr;55(2):523-530. 10.4143/crt.2022.1360.
A Phase II Trial of S-1 and Oxaliplatin in Patients with Metastatic Breast Cancer Previously Treated with Anthracycline and Taxane (KCSG-BR07-03)
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Translational Medicine, Seoul National University College of Medicine, Seoul, Korea
- 4Cancer Research Institute, Seoul National University, Seoul, Korea
- 5Center for Breast Cancer, National Cancer Center, Goyang, Korea
- 6Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Division of Medical Oncology and Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 8Division of Hematology-Oncology, Samsung Medical Center, Seoul, Korea
- 9Department of Internal Medicine, Inha University Hospital, Incheon, Korea
- 10Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 11Department of Hematology and Oncology, Ewha Womans University Medical Center, Seoul, Korea
- 12Division of Hematology-Oncology, Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 13Department of Medical Oncology and Hematology, Kyung Hee University Hospital, Seoul, Korea
- KMID: 2541239
- DOI: http://doi.org/10.4143/crt.2022.1360
Abstract
- Purpose
This single-arm phase II trial investigate the efficacy and safety of S-1 plus oxaliplatin (SOX) in patients with metastatic breast cancer.
Materials and Methods
Patients with metastatic breast cancer previously treated with anthracyclines and taxanes were enrolled. Patients received S-1 (40-60 mg depending on patient’s body surface area, twice a day, day 1-14) and oxaliplatin (130 mg/m2, day 1) in 3 weeks cycle until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) according to Response Evaluation Criteria in Solid Tumor 1.1. Secondary endpoints included time-to-progression (TTP), duration-of-response (DoR), overall survival (OS), and adverse events.
Results
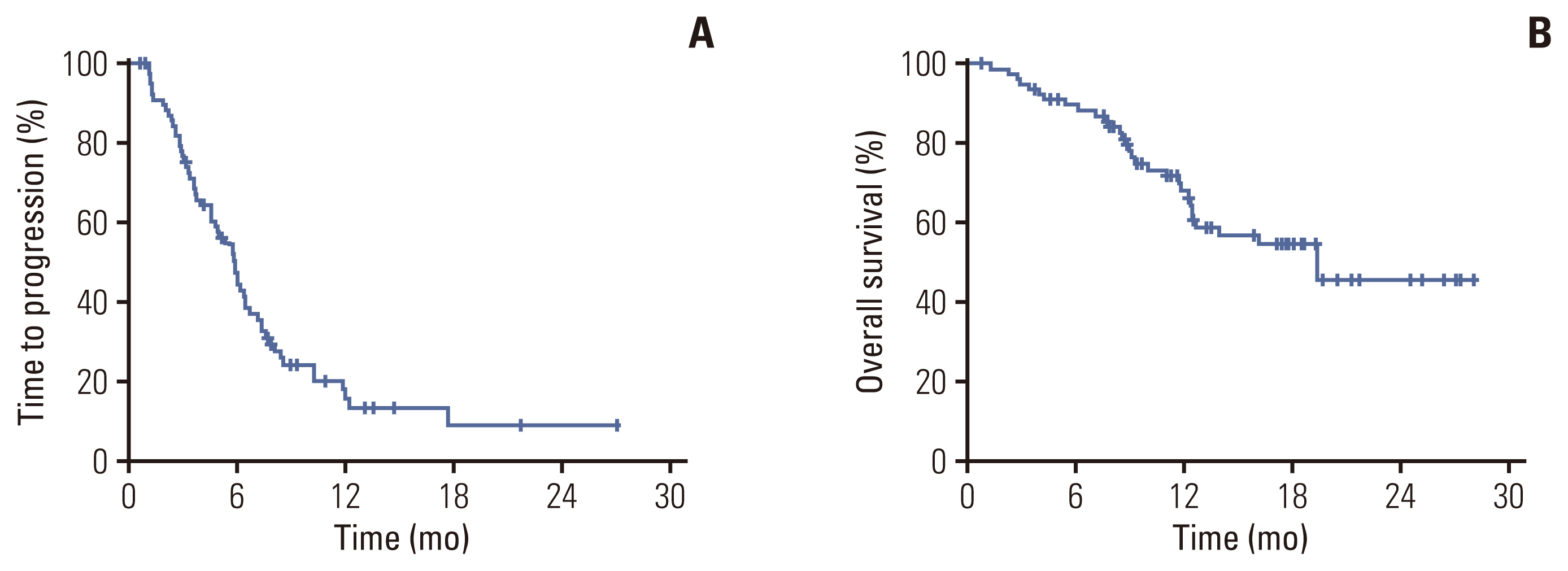
A total of 87 patients were enrolled from 11 institutions in Korea. Hormone receptor was positive in 54 (62.1%) patients and six (6.9%) had human epidermal growth factor receptor 2–positive disease. Forty-eight patients (85.1%) had visceral metastasis and 74 (55.2%) had more than three sites of metastases. The ORR of SOX regimen was 38.5% (95% confidence interval [CI], 26.9 to 50.0) with a median TTP of 6.0 months (95% CI, 5.1 to 6.9). Median DoR and OS were 10.3 months (95% CI, 5.5 to 15.1) and 19.4 (95% CI, not estimated) months, respectively. Grade 3 or 4 neutropenia was reported in 28 patients (32.1%) and thrombocytopenia was observed in 23 patients (26.6%).
Conclusion
This phase II study showed that SOX regimen is a reasonable option in metastatic breast cancer previously treated with anthracyclines and taxanes.
Keyword
Figure
Reference
-
References
1. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019; 69:438–51.
Article2. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020; 31:1623–49.3. Partridge AH, Rumble RB, Carey LA, Come SE, Davidson NE, Di Leo A, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2-negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014; 32:3307–29.
Article4. Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, et al. Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996; 56:2602–6.5. Saek T, Takashima S, Sano M, Horikoshi N, Miura S, Shimizu S, et al. A phase II study of S-1 in patients with metastatic breast cancer: a Japanese trial by the S-1 Cooperative Study Group, Breast Cancer Working Group. Breast Cancer. 2004; 11:194–202.
Article6. Takashima T, Mukai H, Hara F, Matsubara N, Saito T, Takano T, et al. Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol. 2016; 17:90–8.
Article7. Mukai H, Uemura Y, Akabane H, Watanabe T, Park Y, Takahashi M, et al. Anthracycline-containing regimens or taxane versus S-1 as first-line chemotherapy for metastatic breast cancer. Br J Cancer. 2021; 125:1217–25.
Article8. Saeki T, Takashima S, Sano M, Horikoshi N, Miura S, Shimizu S, et al. A late phase II clinical study of S-1 in patients with progressed, refractory breast cancer. Gan To Kagaku Ryoho. 2004; 31:539–47.9. He MM, Wu WJ, Wang F, Wang ZQ, Zhang DS, Luo HY, et al. S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for advanced gastric carcinoma: a meta-analysis. PLoS One. 2013; 8:e82798.
Article10. Kwakman JJ, Simkens LHJ, van Rooijen JM, van de Wouw AJ, Ten Tije AJ, Creemers GJ, et al. Randomized phase III trial of S-1 versus capecitabine in the first-line treatment of metastatic colorectal cancer: SALTO study by the Dutch Colorectal Cancer Group. Ann Oncol. 2017; 28:1288–93.
Article11. Pendyala L, Creaven PJ. In vitro cytotoxicity, protein binding, red blood cell partitioning, and biotransformation of oxaliplatin. Cancer Res. 1993; 53:5970–6.12. Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998; 9:1053–71.
Article13. Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998; 25:4–12.14. Garufi C, Nistico C, Brienza S, Vaccaro A, D’Ottavio A, Zappala AR, et al. Single-agent oxaliplatin in pretreated advanced breast cancer patients: a phase II study. Ann Oncol. 2001; 12:179–82.
Article15. de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938–47.
Article16. Kim DY, Kim JH, Lee SH, Kim TY, Heo DS, Bang YJ, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol. 2003; 14:383–7.
Article17. Zelek L, Cottu P, Tubiana-Hulin M, Vannetzel JM, Chollet P, Misset JL, et al. Phase II study of oxaliplatin and fluorouracil in taxane- and anthracycline-pretreated breast cancer patients. J Clin Oncol. 2002; 20:2551–8.
Article18. Pectasides D, Pectasides M, Farmakis D, Bountouroglou N, Nikolaou M, Koumpou M, et al. Oxaliplatin plus high-dose leucovorin and 5-fluorouracil in pretreated advanced breast cancer: a phase II study. Ann Oncol. 2003; 14:537–42.
Article19. Kim HS, Park MJ, Uhm JE, Lee Y, Lee HY, Kang EM, et al. A phase I trial of S-1 with oxaliplatin in patients with relapsed and metastatic colorectal cancer. Int J Colorectal Dis. 2009; 24:1311–6.
Article20. Park I, Lee JL, Ryu MH, Chang HM, Kim TW, Sym SJ, et al. Phase I/II and pharmacokinetic study of S-1 and oxaliplatin in previously untreated advanced gastric cancer. Cancer Che-mother Pharmacol. 2010; 65:473–80.
Article21. Liu J, Xiao Y, Wei W, Guo JX, Liu YC, Huang XH, et al. Clinical efficacy of administering oxaliplatin combined with S-1 in the treatment of advanced triple-negative breast cancer. Exp Ther Med. 2015; 10:379–85.
Article22. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.23. Sparano JA, Vrdoljak E, Rixe O, Xu B, Manikhas A, Medina C, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010; 28:3256–63.
Article24. Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999; 17:485–93.
Article25. Fumoleau P, Largillier R, Clippe C, Dieras V, Orfeuvre H, Lesimple T, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004; 40:536–42.
Article26. Yamamoto D, Iwase S, Tsubota Y, Ariyoshi K, Kawaguchi T, Miyaji T, et al. Randomized study of orally administered fluorinated pyrimidines (capecitabine versus S-1) in women with metastatic or recurrent breast cancer: Japan Breast Cancer Research Network 05 Trial. Cancer Chemother Pharmacol. 2015; 75:1183–9.
Article27. Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015; 26:141–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Study of Gemcitabine Monotherapy in Breast Cancer Patients Refractory to Anthracycline and Taxane
- Treatment with Cisplatin and Etoposide Chemotherapy in Patient with Metastatic Breast Cancer
- Phase II Study of Gemcitabine plus Cisplatin in Patients with Anthracycline- and Taxane- Pretreated Metastatic Breast Cancer
- Gemcitabine and Vinorelbine Combination Chemotherapy in Anthracycline- and Taxane-pretreated Advanced Breast Cancer
- Capecitabine Monotherapy in Taxane-Refractory Metastatic Breast Cancer (MBC) Patients