Ann Surg Treat Res.
2023 Apr;104(4):183-194. 10.4174/astr.2023.104.4.183.
Long-term outcomes of liver transplantation using grafts from donors with active hepatitis B virus replication: a multicenter cohort study
- Affiliations
-
- 1Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 3Department of Surgery, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Korea
- 4Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2541144
- DOI: http://doi.org/10.4174/astr.2023.104.4.183
Abstract
- Purpose
Liver grafts from donors with HBV infection contributed to expanding the donor pool under the hepatitis B immunoglobulin and antiviral agents (nucleos(t)ide analogues) in the HBV-endemic area. We report long-term outcomes of liver transplantations (LTs) using grafts from donors with active or chronic HBV infection.
Methods
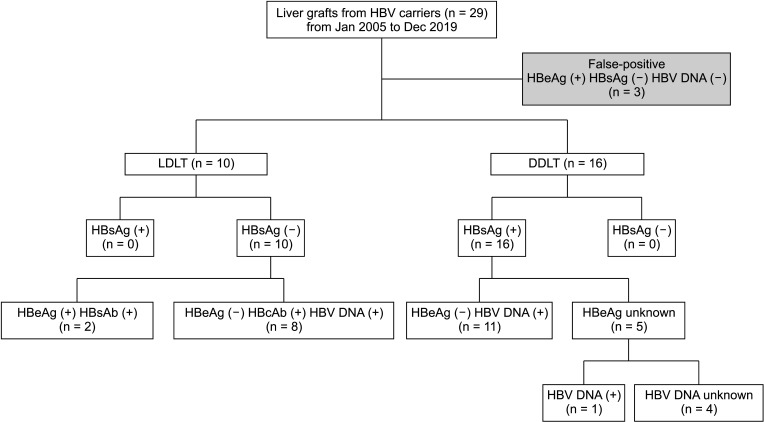
Overall, 2,260 LTs performed in 3 major hospitals in Seoul from January 2000 to April 2019 were assessed for inclusion. Twenty-six grafts (1.2%) were obtained from HBsAg (+), HBeAb (+), or HBcAb (+) donors, and recipient outcomes were retrospectively reviewed. Donor and recipient demographics and transplantation outcomes were analyzed.
Results
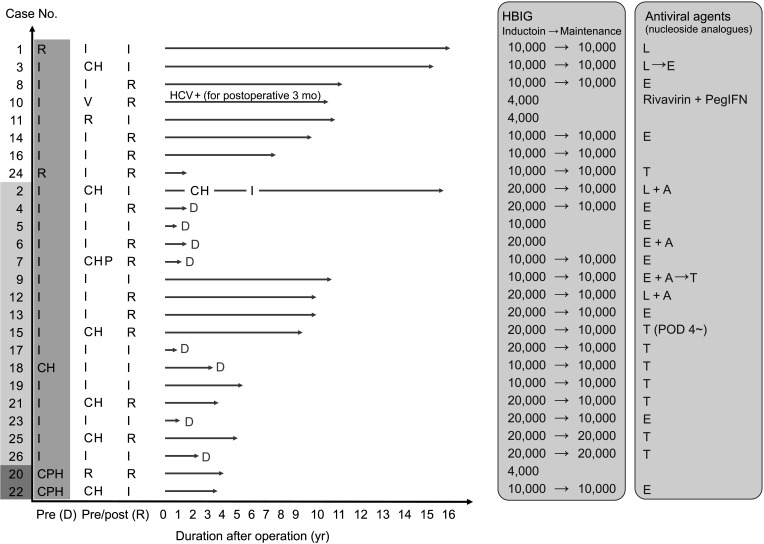
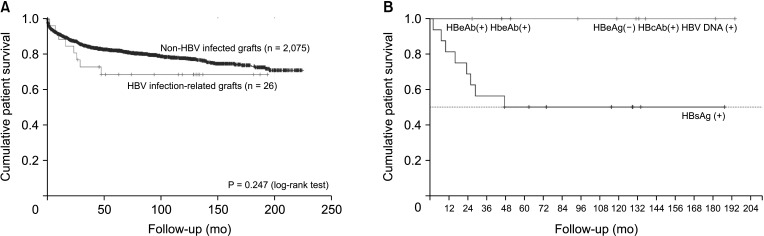
Sixteen deceased donor LTs were performed using active HBsAg (+) grafts. Ten other LTs were sourced from 10 living donors. There was no significant difference in survival in patients who received deceased donor LTs compared with that in those who underwent LT with non–hepatitis virus-infected grafts. Fourteen patients who were followed up for >5 years were stable, and no difference in hepatocellular carcinoma recurrence rate was observed 5 years after transplantation between transplants from donors with and those without HBV.
Conclusion
Considering long-term outcomes, liver grafts from donors with active HBV replication can be safely used for LT.
Figure
Reference
-
1. Lee WC, Chou HS, Wu TJ, Lee CS, Lee CF, Chan KM. Indicators and outcome of liver transplantation in acute liver decompensation after flares of hepatitis B. J Viral Hepat. 2011; 18:193–199. PMID: 20367797.2. Chan KM, Yu MC, Chou HS, Wu TJ, Lee CF, Lee WC. Significance of tumor necrosis for outcome of patients with hepatocellular carcinoma receiving locoregional therapy prior to liver transplantation. Ann Surg Oncol. 2011; 18:2638–2646. PMID: 21584831.3. British Transplantation Society (BTS). Guidelines for hepatitis B & solid organ transplantation. BTS;2018.4. Hashimoto K, Miller C. The use of marginal grafts in liver transplantation. J Hepatobiliary Pancreat Surg. 2008; 15:92–101. PMID: 18392701.5. Lan X, Zhang H, Li HY, Chen KF, Liu F, Wei YG, et al. Feasibility of using marginal liver grafts in living donor liver transplantation. World J Gastroenterol. 2018; 24:2441–2456. PMID: 29930466.6. Yim SY, Kim JH. The epidemiology of hepatitis B virus infection in Korea. Korean J Intern Med. 2019; 34:945–953. PMID: 30919608.7. Loggi E, Micco L, Ercolani G, Cucchetti A, Bihl FK, Grazi GL, et al. Liver transplantation from hepatitis B surface antigen positive donors: a safe way to expand the donor pool. J Hepatol. 2012; 56:579–585. PMID: 22027583.8. Kim MS, Lee KW, Hwang S, Kwon CH, You YK, Nah YW, et al. Research for modification of emergency status in deceased donor liver allocation: survival analysis of waiting patients for liver transplantation. J Korean Soc Transplant. 2014; 28:59–68.9. Huprikar S, Danziger-Isakov L, Ahn J, Naugler S, Blumberg E, Avery RK, et al. Solid organ transplantation from hepatitis B virus-positive donors: consensus guidelines for recipient management. Am J Transplant. 2015; 15:1162–1172. PMID: 25707744.10. Samuel D, Bismuth A, Mathieu D, Arulnaden JL, Reynes M, Benhamou JP, et al. Passive immunoprophylaxis after liver transplantation in HBsAg-positive patients. Lancet. 1991; 337:813–815. PMID: 1672913.11. Chou HS, Cheng CH, Hung HC, Lee JC, Wang YC, Wu TH, et al. Significance of hepatitis B recurrence in liver transplantation recipients. Biomed Res Int. 2020; 2020:2489526. PMID: 32934957.12. Lee WC, Chou HS, Lee CS, Wu TH, Wang YC, Cheng CH, et al. Viral activity and outcome of hepatitis B surface antigen-positive grafts in deceased liver transplantation. J Viral Hepat. 2018; 25:874–877. PMID: 29431877.13. Shouval D. Expanding the donor pool for liver transplant recipients using HBsAg positive grafts. J Hepatol. 2014; 61:717–719. PMID: 25038488.14. Zhang JA, Kwee SA, Wong LL. Late recurrence of hepatocellular carcinoma after liver transplantation. Hepatoma Res. 2017; 3:58–66. PMID: 28966983.15. Shaw BI, Lucander A, Ravindra KV. Very late recurrence of hepatocellular carcinoma after orthotopic liver transplantation: presentation and management. Transplant Direct. 2019; 5:e483. PMID: 31579811.16. Korean Association for the Study of the Liver. 2022 Chronic hepatitis B treatment guidelines of Korean Association for the Study of the Liver: management and treatment of chronic hepatitis B [Internet]. Korean Association for the Study of the Liver;2022. cited 2023 Jan 6. Available from: https://www.kasl.org/bbs/skin/guide/download.php?code=guide&number=15609 .17. Yu S, Yu J, Zhang W, Cheng L, Ye Y, Geng L, et al. Safe use of liver grafts from hepatitis B surface antigen positive donors in liver transplantation. J Hepatol. 2014; 61:809–815. PMID: 24824283.18. Nasir M, Wu GY. Prevention of HBV recurrence after liver transplant: a review. J Clin Transl Hepatol. 2020; 8:150–160. PMID: 32832395.19. Fung J, Chan SC, Cheung C, Yuen MF, Chok KS, Sharr W, et al. Oral nucleoside/nucleotide analogs without hepatitis B immune globulin after liver transplantation for hepatitis B. Am J Gastroenterol. 2013; 108:942–948. PMID: 23629601.20. Duvoux C, Belli LS, Fung J, Angelico M, Buti M, Coilly A, et al. 2020 position statement and recommendations of the European Liver and Intestine Transplantation Association (ELITA): management of hepatitis B virus-related infection before and after liver transplantation. Aliment Pharmacol Ther. 2021; 54:583–605. PMID: 34287994.21. Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010; 53:20–28. PMID: 20068337.22. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 2019; 25:93–159. PMID: 31185710.23. Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019; 18:827–844. PMID: 31455905.24. Saab S, Desai S, Tsaoi D, Durazo F, Han S, McClune A, et al. Posttransplantation hepatitis B prophylaxis with combination oral nucleoside and nucleotide analog therapy. Am J Transplant. 2011; 11:511–517. PMID: 21299826.25. Muhammad H, Tehreem A, Hammami MB, Ting PS, Idilman R, Gurakar A. Hepatitis D virus and liver transplantation: indications and outcomes. World J Hepatol. 2021; 13:291–299. PMID: 33815673.26. Botea F, Brasoveanu V, Dima S, Iacob S, Droc G, Ionescu M, et al. Influence of hepatitis D virus co-infection on graft survival after liver transplantation for hepatitis B virus cirrhosis: abstract# B1122. Transplantation. 2014; 98:736.27. Bahde R, Hölzen JP, Wolters HH, Schmidt HH, Bock CT, Lügering A, et al. Course of a HBsAg positive liver transplantation in a hepatitis B and D virus coinfected recipient. Ann Hepatol. 2011; 10:355–360. PMID: 21677340.28. Saab S, Yeganeh M, Nguyen K, Durazo F, Han S, Yersiz H, et al. Recurrence of hepatocellular carcinoma and hepatitis B reinfection in hepatitis B surface antigen-positive patients after liver transplantation. Liver Transpl. 2009; 15:1525–1534. PMID: 19877207.29. Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017; 266:118–125. PMID: 27433914.30. Faria LC, Gigou M, Roque-Afonso AM, Sebagh M, Roche B, Fallot G, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008; 134:1890–1899. PMID: 18424269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Hepatitis B Virus DNA in Liver Grafts Obtained from HBsAb and HBcAb Positive Organ Donors
- Prophylaxis for Hepatitis B Core Antibody-Positive Donors after Liver Transplantation
- Cost-effectiveness and long-term outcomes of liver transplantation using hepatitis B core antibody-positive grafts with hepatitis B immunoglobulin prophylaxis in Korea
- Long-term outcomes of liver transplantation using grafts from donors with active and chronic hepatitis B virus infection
- Comparison of clinical outcomes using the left and right liver grafts in adult-to-adult living donor liver transplantation: a retrospective cohort study using the Korean Organ Transplantation Registry