Korean J Transplant.
2022 Dec;36(4):283-288. 10.4285/kjt.22.0027.
Eculizumab as rescue therapy in a kidney transplant recipient with atypical hemolytic uremic syndrome: a case report
- Affiliations
-
- 1Department of Surgery, Korea University Anam Hospital, Seoul, Korea
- 2Department of Rehabilitation Medicine, Korea University Anam Hospital, Seoul, Korea
- 3Department of Pathology, Korea University Anam Hospital, Seoul, Korea
- 4Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea
- KMID: 2537540
- DOI: http://doi.org/10.4285/kjt.22.0027
Abstract
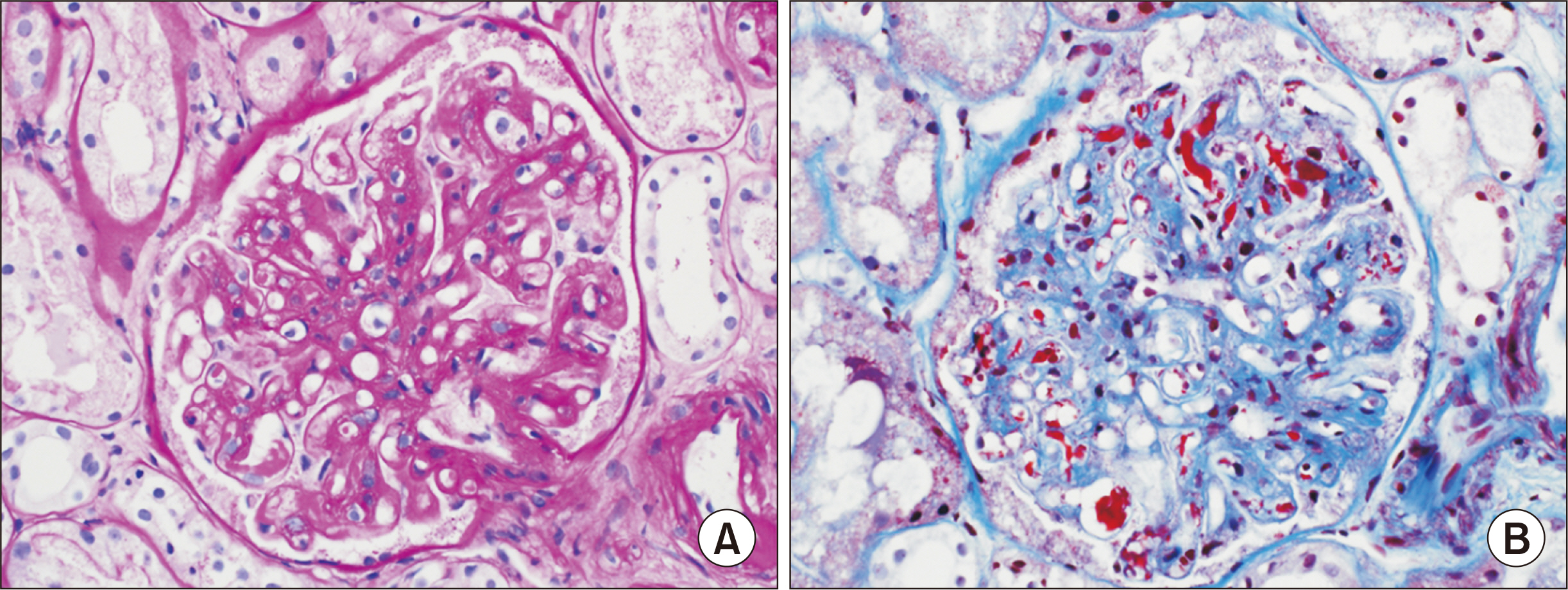
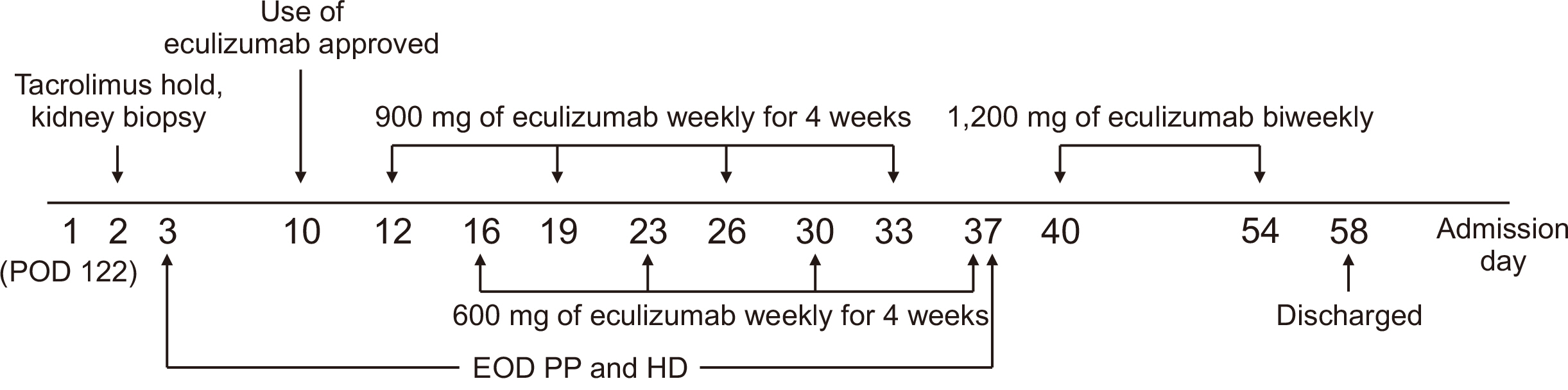
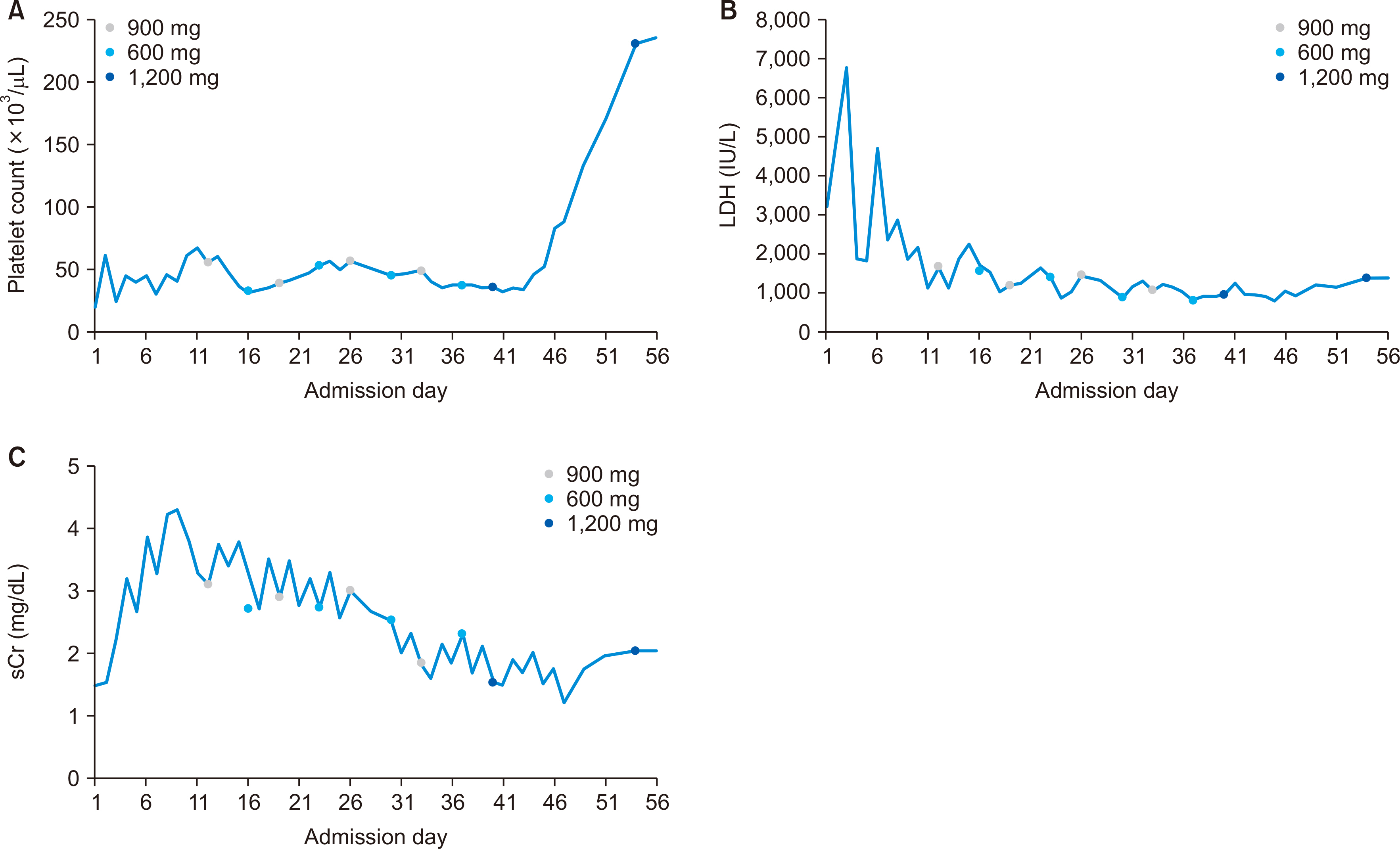
- A 61-year-old female patient with chronic kidney disease due to diabetes mellitus and hypertension–induced nephropathy received a deceased donor kidney transplant in March 2020. In July 2020, she was transferred from a local hospital due to the exacerbation of general weakness and diarrhea. Upon her arrival, we noticed a high level of serum creatinine (sCr) of 1.5 mg/dL and a decrease in urine output. Her laboratory results indicated significant hemolysis, with a hemoglobin level of 7.0 g/dL, platelet count of 20 ×103/μL, and a lactate dehydrogenase level of 3,207 IU/L. Kidney biopsy showed severe thrombotic microangiopathy without any evidence of acute rejection. Under the impression of atypical hemolytic uremic syndrome (aHUS), we immediately started plasmapheresis and hemodialysis for anuria. Eculizumab was considered as a kidney graft rescue therapy since her sCr level was not effectively decreased, and her anuria continued despite hemodialysis and plasmapheresis. Eculizumab (900 mg) was administered weekly for 4 weeks. An additional 600 mg of eculizumab was administered on the day of plasmapheresis. Since the patient’s laboratory data gradually improved, hemodialysis and plasmapheresis were ceased on admission day 37. After that, eculizumab was administered biweekly (1,200 mg) two more times. The patient’s sCr and platelet count normalized after 2 months of eculizumab treatment. Based on our experience, a shorter interval between the clinical diagnosis of aHUS and administration of eculizumab increases the likelihood of rescuing the kidney.
Figure
Reference
-
1. Portoles J, Huerta A, Arjona E, Gavela E, Agüera M, Jiménez C, et al. 2020; Characteristics, management and outcomes of atypical haemolytic uraemic syndrome in kidney transplant patients: a retrospective national study. Clin Kidney J. 14:1173–80. DOI: 10.1093/ckj/sfaa096. PMID: 33841863. PMCID: PMC8023214.2. Alasfar S, Alachkar N. 2014; Atypical hemolytic uremic syndrome post-kidney transplantation: two case reports and review of the literature. Front Med (Lausanne). 1:52. DOI: 10.3389/fmed.2014.00052. PMID: 25593925. PMCID: PMC4292050.3. Zuber J, Le Quintrec M, Sberro-Soussan R, Loirat C, Frémeaux-Bacchi V, Legendre C. 2011; New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol. 7:23–35. DOI: 10.1038/nrneph.2010.155. PMID: 21102542.4. Reynolds JC, Agodoa LY, Yuan CM, Abbott KC. 2003; Thrombotic microangiopathy after renal transplantation in the United States. Am J Kidney Dis. 42:1058–68. DOI: 10.1016/j.ajkd.2003.07.008. PMID: 14582050.5. Zarifian A, Meleg-Smith S, O'donovan R, Tesi RJ, Batuman V. 1999; Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int. 55:2457–66. DOI: 10.1046/j.1523-1755.1999.00492.x. PMID: 10354295.6. Noris M, Remuzzi G. 2010; Thrombotic microangiopathy after kidney transplantation. Am J Transplant. 10:1517–23. DOI: 10.1111/j.1600-6143.2010.03156.x. PMID: 20642678.7. Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, et al. 2013; Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 368:2169–81. DOI: 10.1056/NEJMoa1208981. PMID: 23738544.8. Cavero T, Rabasco C, López A, Román E, Ávila A, Sevillano Á, et al. 2017; Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 32:466–74. DOI: 10.1093/ndt/gfw453. PMID: 28339660. PMCID: PMC5410989.9. Ponticelli C. 2007; De novo thrombotic microangiopathy. An underrated complication of renal transplantation. Clin Nephrol. 67:335–40. DOI: 10.5414/CNP67335. PMID: 17598367.10. Garg N, Rennke HG, Pavlakis M, Zandi-Nejad K. 2018; De novo thrombotic microangiopathy after kidney transplantation. Transplant Rev (Orlando). 32:58–68. DOI: 10.1016/j.trre.2017.10.001. PMID: 29157988.11. Le Quintrec M, Lionet A, Kamar N, Karras A, Barbier S, Buchler M, et al. 2008; Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 8:1694–701. DOI: 10.1111/j.1600-6143.2008.02297.x. PMID: 18557729.12. Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, et al. 2013; Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 8:554–62. DOI: 10.2215/CJN.04760512. PMID: 23307876. PMCID: PMC3613948.13. Karthikeyan V, Parasuraman R, Shah V, Vera E, Venkat KK. 2003; Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant. 3:1289–94. DOI: 10.1046/j.1600-6143.2003.00222.x. PMID: 14510703.14. US Food and Drug Administration. 2018. Drug administration Solaris (eculizumab) [prescribing information]. Alexion Pharmaceuticals;Boston, MA:15. Health Insurance Review Assessment Service (HIRA). 2018. HIRA notification: detailed evaluation criteria for new drugs subject to negotiation [Internet]. HIRA;Seoul: Available from: http://www.hira.or.kr. cited 2022 Apr 20.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The first successful eculizumab rescue therapy of a kidney transplant recipient with atypical hemolytic uremic syndrome in South Korea: a case report
- Atypical hemolytic uremic syndrome and eculizumab therapy in children
- Rapid Resolution of Atypical Hemolytic Uremic Syndrome by Eculizumab Treatment
- Eculizumab therapy for recurrent atypical hemolytic uremic syndrome after kidney transplantation in atypical hemolytic uremic syndrome: a case report
- An abscess formation on the transplanted graft and its successfully treatment in kidney transplantation recipient with de novo atypical hemolytic uremic syndrome treated with eculizumab: a case report