Ann Rehabil Med.
2022 Dec;46(6):292-302. 10.5535/arm.22100.
Hemodynamic Consideration in Intraoperative Neurophysiological Monitoring in Neuromuscular Scoliosis Surgery
- Affiliations
-
- 1Department of Rehabilitation Medicine, Gangnam Severance Hospital, Rehabilitation Institute of Neuromuscular Disease, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Orthopedic Surgery, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2537160
- DOI: http://doi.org/10.5535/arm.22100
Abstract
Objective
To prove the hypothesis that the parameters of intraoperative neurophysiological monitoring (IONM) during will be more deteriorated in neuromuscular scoliosis (NMS) than in adolescent idiopathic scoliosis (AIS).
Methods
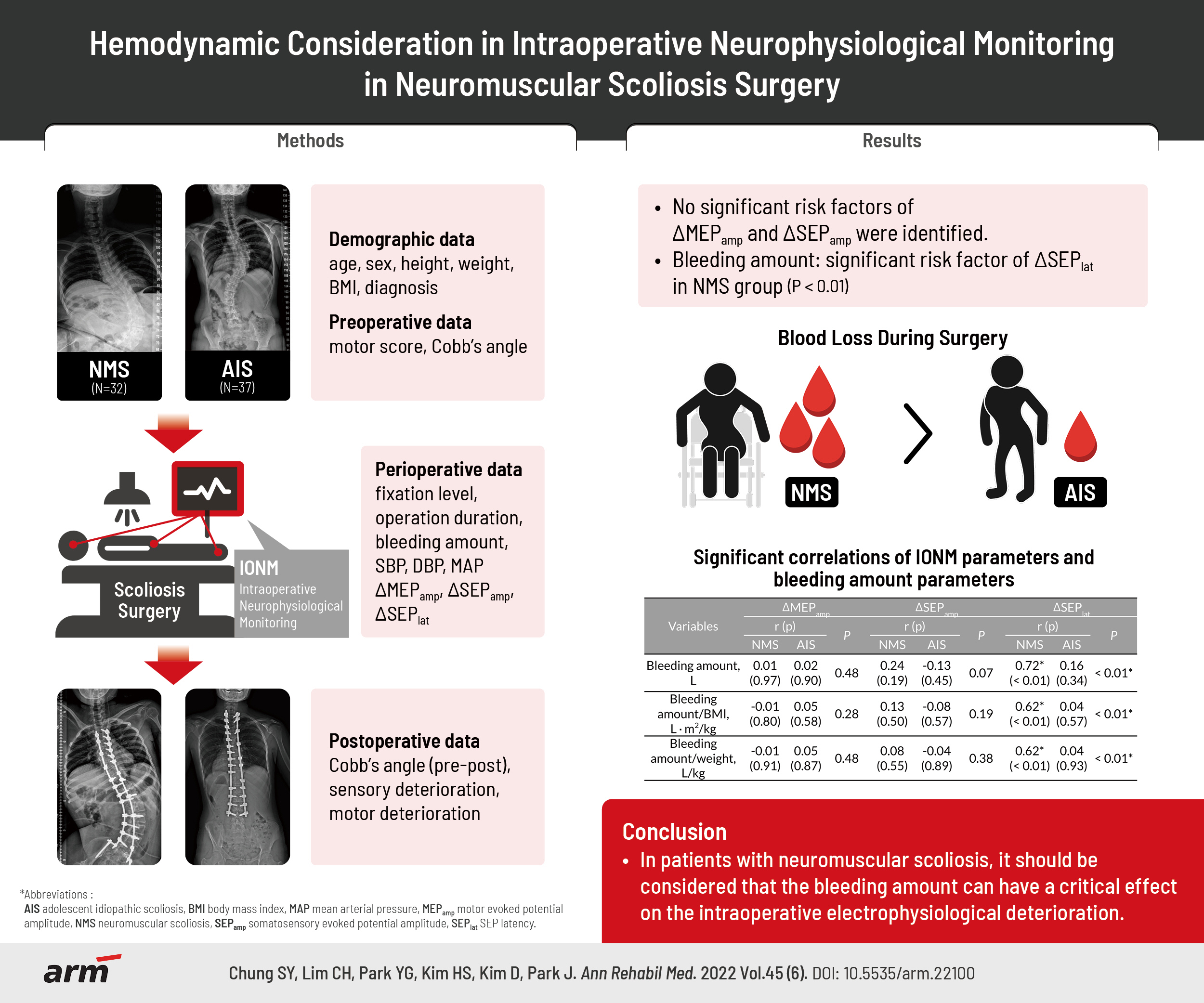
This retrospective study reviewed the data of 69 patients (NMS=32, AIS=37) who underwent scoliosis surgery under IONM. The amplitude of motor evoked potentials (MEPs), and the amplitude and the latency of somatosensory evoked potentials (SEPs) were examined. Demographic, preoperative, perioperative and postoperative data were analyzed to determine whether they affected the IONM parameters for each group.
Results
Of the items analyzed, the bleeding amount was the only significant risk factor for SEP latency deterioration in the NMS group only. The amplitude of SEP and MEP did not correlate with the hemodynamic parameters. The NMS/AIS ratios of the bleeding-related parameters were higher in the order of bleeding amount/weight (2.62, p<0.01), bleeding amount/body mass index (2.13, p<0.01), and bleeding amount (1.56, p<0.01). This study suggests that SEP latency is more vulnerable than SEP or MEP amplitude in ischemic conditions during scoliosis surgery.
Conclusion
In NMS patients, it should be considered that the bleeding amount can have a critical effect on intraoperative electrophysiological deterioration.
Figure
Reference
-
1. Molnar GE, Alexander MA. Pediatric rehabilitation, 3rd ed. Philadelphia, PA: Hanley & Belfus;1999.2. Murphy NA, Firth S, Jorgensen T, Young PC. Spinal surgery in children with idiopathic and neuromuscular scoliosis: what’s the difference? J Pediatr Orthop. 2006; 26:216–20.3. Romero-Munoz LM, Segura-Fragoso A, Talavera-Diaz F, Guimbard-Perez J, Caba-Mora D, Barriga-Martin A. Neurological injury as a complication of spinal surgery: incidence, risk factors, and prognosis. Spinal Cord. 2020; 58:318–23.4. Diab M, Smith AR, Kuklo TR; Spinal Deformity Study Group. Neural complications in the surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2007; 32:2759–63.5. Korean Society of Intraoperative Neurophysiological Monitoring; Korean Neurological Association; Korean Academy of Rehabilitation Medicine; Korean Society of Clinical Neurophysiology; Korean Association of EMG Electrodiagnostic Medicine. Clinical practice guidelines for intraoperative neurophysiological monitoring: 2020 update. Ann Clin Neurophysiol. 2021; 23:35–45.6. Yu T, Li QJ, Zhang XW, Wang Y, Jiang QY, Zhu XJ, et al. Multimodal intraoperative monitoring during surgical correction of scoliosis to avoid neurologic damage. Medicine (Baltimore). 2019; 98:e15067.7. Mendiratta A, Emerson RG. Neurophysiologic intraoperative monitoring of scoliosis surgery. J Clin Neurophysiol. 2009; 26:62–9.8. MacDonald DB, Al Zayed Z, Khoudeir I, Stigsby B. Monitoring scoliosis surgery with combined multiple pulse transcranial electric motor and cortical somatosensory-evoked potentials from the lower and upper extremities. Spine (Phila Pa 1976). 2003; 28:194–203.9. Chang SH, Park YG, Kim DH, Yoon SY. Monitoring of motor and somatosensory evoked potentials during spine surgery: intraoperative changes and postoperative outcomes. Ann Rehabil Med. 2016; 40:470–80.10. Pastorelli F, Di Silvestre M, Plasmati R, Michelucci R, Greggi T, Morigi A, et al. The prevention of neural complications in the surgical treatment of scoliosis: the role of the neurophysiological intraoperative monitoring. Eur Spine J. 2011; 20(Suppl 1):S105–14.11. Thirumala PD, Huang J, Thiagarajan K, Cheng H, Balzer J, Crammond DJ. Diagnostic accuracy of combined multimodality somatosensory evoked potential and transcranial motor evoked potential intraoperative monitoring in patients with idiopathic scoliosis. Spine (Phila Pa 1976). 2016; 41:E1177–84.12. Pastorelli F, Di Silvestre M, Vommaro F, Maredi E, Morigi A, Bacchin MR, et al. Intraoperative monitoring of somatosensory (SSEPs) and transcranial electric motor-evoked potentials (tce-MEPs) during surgical correction of neuromuscular scoliosis in patients with central or peripheral nervous system diseases. Eur Spine J. 2015; 24 Suppl 7:931–6.13. Pankowski R, Dziegiel K, Roclawski M, Smoczynski A, Ceynowa M, Kloc W, et al. Intraoperative neurophysiologic monitoring (INM) in scoliosis surgery. Stud Health Technol Inform. 2012; 176:319–21.14. Branston NM, Symon L, Crockard HA, Pasztor E. Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol. 1974; 45:195–208.15. Ngai AC, Jolley MA, D’Ambrosio R, Meno JR, Winn HR. Frequency-dependent changes in cerebral blood flow and evoked potentials during somatosensory stimulation in the rat. Brain Res. 1999; 837:221–8.16. Fehlings MG, Tator CH, Linden RD. The relationships among the severity of spinal cord injury, motor and somatosensory evoked potentials and spinal cord blood flow. Electroencephalogr Clin Neurophysiol. 1989; 74:241–59.17. Banoub M, Tetzlaff JE, Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials: implications for perioperative monitoring. Anesthesiology. 2003; 99:716–37.18. McPherson RW, Zeger S, Traystman RJ. Relationship of somatosensory evoked potentials and cerebral oxygen consumption during hypoxic hypoxia in dogs. Stroke. 1986; 17:30–6.19. Nielsen VK, Kardel T. Temporospatial effects on orthodromic sensory potential propagation during ischemia. Ann Neurol. 1981; 9:597–604.20. Maruhashi J, Wright EB. Effect of oxygen lack on the single isolated mammalian (rat) nerve fiber. J Neurophysiol. 1967; 30:434–52.21. Seyal M, Mull B. Mechanisms of signal change during intraoperative somatosensory evoked potential monitoring of the spinal cord. J Clin Neurophysiol. 2002; 19:409–15.22. Reuter DG, Tacker WA, Badylak SF, Voorhees WD 3rd, Konrad PE. Correlation of motor-evoked potential response to ischemic spinal cord damage. J Thorac Cardiovasc Surg. 1992; 104:262–72.23. Lesnick JE, Michele JJ, Simeone FA, DeFeo S, Welsh FA. Alteration of somatosensory evoked potentials in response to global ischemia. J Neurosurg. 1984; 60:490–4.24. Ji Y, Meng B, Yuan C, Yang H, Zou J. Monitoring somatosensory evoked potentials in spinal cord ischemia-reperfusion injur y. Neural Regen Res. 2013; 8:3087–94.25. Schwartz DM, Auerbach JD, Dormans JP, Flynn J, Drummond DS, Bowe JA, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007; 89:2440–9.26. Fievez E, Schultze-Balin C, Herbaux B, Dalmas S, Scherpereel P. A study of blood loss during surgery for scoliosis: posterior approach in 319 adolescents. Cah Anesthesiol. 1995; 43:425–33.27. Kannan S, Meert KL, Mooney JF, Hillman-Wiseman C, Warrier I. Bleeding and coagulation changes during spinal fusion surgery: a comparison of neuromuscular and idiopathic scoliosis patients. Pediatr Crit Care Med. 2002; 3:364–9.28. Masood SA, Kazmouz S, Heydemann P, Li H, Kenny D. Under-recognition of low blood pressure readings in patients with Duchenne muscular dystrophy. Pediatr Cardiol. 2015; 36:1489–94.29. Marui FR, Bianco HT, Bombig MT, Palmeira NG, Thalenberg JM, Povoa FF, et al. Behavior of blood pressure variables in children and adolescents with Duchenne muscular dystrophy. Arq Bras Cardiol. 2018; 110:551–7.30. Schmidt GN, Burmeister MA, Lilje C, Wappler F, Bischoff P. Acute heart failure during spinal surgery in a boy with Duchenne muscular dystrophy. Br J Anaesth. 2003; 90:800–4.31. Carreon LY, Puno RM, Lenke LG, Richards BS, Sucato DJ, Emans JB, et al. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2007; 89:2427–32.32. Jia R, Li N, Xu BY, Zhang W, Gu XP, Ma ZL. Incidence, influencing factors, and prognostic impact of intraoperative massive blood loss in adolescents with neuromuscular scoliosis: a STROBE-compliant retrospective observational analysis. Medicine (Baltimore). 2017; 96:e6292.33. Othman Z, Lenke LG, Bolon SM, Padberg A. Hypotension-induced loss of intraoperative monitoring data during surgical correction of Scheuermann kyphosis: a case report. Spine (Phila Pa 1976). 2004; 29:E258–65.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical practice guidelines for intraoperative neurophysiological monitoring: 2020 update
- Preservation of the superior laryngeal nerve without neuromonitoring during thyroid surgery
- Intraoperative Neurophysiological Monitoring for Spinal Cord Tumor Surgery: Comparison of Motor and Somatosensory Evoked Potentials According to Tumor Types
- Anesthetic experience performing intraoperative monitoring of motor evoked potentials during scoliosis surgery in adolescent patients: report on 7 cases: Seven cases report
- Comparing the effects of vecuronium and cisatracurium on electrophysiologic monitoring during neurosurgery: a randomized controlled study