Korean J Physiol Pharmacol.
2022 Nov;26(6):519-530. 10.4196/kjpp.2022.26.6.519.
Lactate promotes vascular smooth muscle cell switch to a synthetic phenotype by inhibiting miR-23b expression
- Affiliations
-
- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Xi'an Jiaotong University, Shaanxi 710004, China
- KMID: 2534527
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.519
Abstract
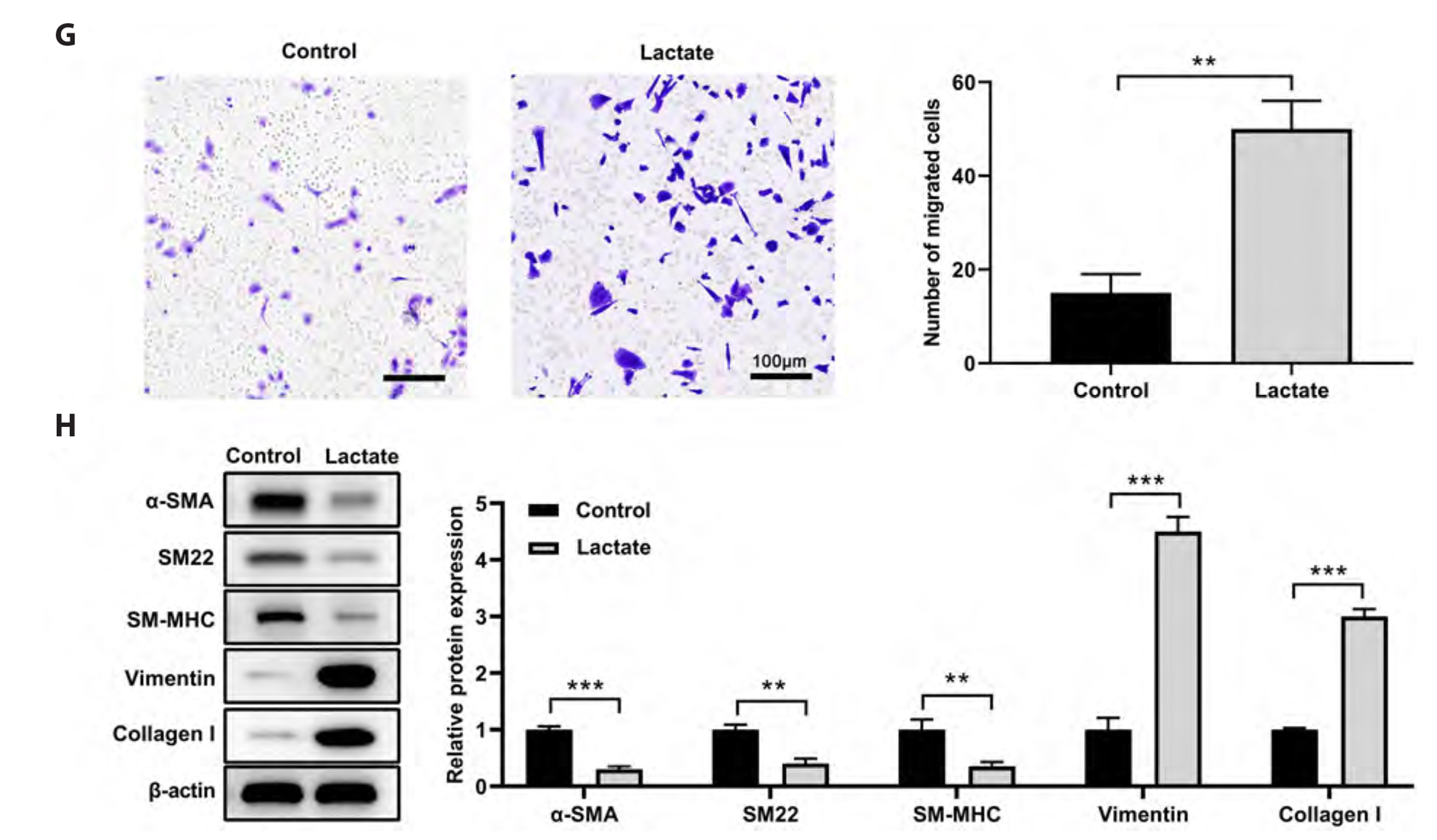
- Recent research indicates that lactate promotes the switching of vascular smooth muscle cells (VSMCs) to a synthetic phenotype, which has been implicated in various vascular diseases. This study aimed to investigate the effects of lactate on the VSMC phenotype switch and the underlying mechanism. The CCK-8 method was used to assess cell viability. The microRNAs and mRNAs levels were evaluated using quantitative PCR. Targets of microRNA were predicted using online tools and confirmed using a luciferase reporter assay. We found that lactate promoted the switch of VSMCs to a synthetic phenotype, as evidenced by an increase in VSMC proliferation, mitochondrial activity, migration, and synthesis but a decrease in VSMC apoptosis. Lactate inhibited miR-23b expression in VSMCs, and miR-23b inhibited VSMC's switch to the synthetic phenotype. Lactate modulated the VSMC phenotype through downregulation of miR-23b expression, suggesting that overexpression of miR-23b using a miR-23b mimic attenuated the effects of lactate on VSMC phenotype modulation. Moreover, we discovered that SMAD family member 3 (SMAD3) was the target of miR-23b in regulating VSMC phenotype. Further findings suggested that lactate promotes VSMC switch to synthetic phenotype by targeting SMAD3 and downregulating miR-23b. These findings suggest that correcting the dysregulation of miR-23b/ SMAD3 or lactate metabolism is a potential treatment for vascular diseases.
Keyword
Figure
Reference
-
1. Basatemur GL, Jørgensen HF, Clarke MCH, Bennett MR, Mallat Z. 2019; Vascular smooth muscle cells in atherosclerosis. Nat Rev Cardiol. 16:727–744. DOI: 10.1038/s41569-019-0227-9. PMID: 31243391. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068318311&origin=inward.
Article2. Bennett MR, Sinha S, Owens GK. 2016; Vascular smooth muscle cells in atherosclerosis. Circ Res. 118:692–702. DOI: 10.1161/CIRCRESAHA.115.306361. PMID: 26892967. PMCID: PMC4762053. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84959048825&origin=inward.
Article3. Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O'Brien C, Walls D, Redmond EM, Cahill PA. 2008; Notch and vascular smooth muscle cell phenotype. Circ Res. 103:1370–1382. DOI: 10.1161/CIRCRESAHA.108.187534. PMID: 19059839. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=58149379347&origin=inward.
Article4. Lacolley P, Regnault V, Avolio AP. 2018; Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res. 114:513–528. DOI: 10.1093/cvr/cvy009. PMID: 29514201. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042943530&origin=inward.
Article5. Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, De Meyer GRY. 2018; Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 114:622–634. DOI: 10.1093/cvr/cvy007. PMID: 29360955. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042944408&origin=inward.
Article6. Chistiakov DA, Orekhov AN, Bobryshev YV. 2015; Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 214:33–50. DOI: 10.1111/apha.12466. PMID: 25677529. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84927640251&origin=inward.
Article7. Salabei JK, Hill BG. 2015; Autophagic regulation of smooth muscle cell biology. Redox Biol. 4:97–103. DOI: 10.1016/j.redox.2014.12.007. PMID: 25544597. PMCID: PMC4309847. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84919782591&origin=inward.
Article8. Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. 2007; Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 21:2602–2612. DOI: 10.1096/fj.07-8174com. PMID: 17395833. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34547790342&origin=inward.
Article9. Hirschhaeuser F, Sattler UG, Mueller-Klieser W. 2011; Lactate: a metabolic key player in cancer. Cancer Res. 71:6921–6925. DOI: 10.1158/0008-5472.CAN-11-1457. PMID: 22084445. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=81155126061&origin=inward.
Article10. Dhup S, Dadhich RK, Porporato PE, Sonveaux P. 2012; Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 18:1319–1330. DOI: 10.2174/138161212799504902. PMID: 22360558. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84857716739&origin=inward.
Article11. Müller P, Duderstadt Y, Lessmann V, Müller NG. 2020; Lactate and BDNF: key mediators of exercise induced neuroplasticity? J Clin Med. 9:1136. DOI: 10.3390/jcm9041136. PMID: 32326586. PMCID: PMC7230639. PMID: 76c733ff1dc9428887e259897e30f192. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85087787055&origin=inward.
Article12. Baltazar F, Afonso J, Costa M, Granja S. 2020; Lactate beyond a waste metabolite: metabolic affairs and signaling in malignancy. Front Oncol. 10:231. DOI: 10.3389/fonc.2020.00231. PMID: 32257942. PMCID: PMC7093491. PMID: 2b0659710b5a4cafb82419c662eae7cc. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082649332&origin=inward.
Article13. Pereira-Nunes A, Afonso J, Granja S, Baltazar F. 2020; Lactate and lactate transporters as key players in the maintenance of the Warburg effect. Adv Exp Med Biol. 1219:51–74. DOI: 10.1007/978-3-030-34025-4_3. PMID: 32130693. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85081043083&origin=inward.
Article14. Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, Geng Z, Johnson C, Young B, Henke C, Gourley GR, Zhang J. 2017; Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res. 121:1251–1262. DOI: 10.1161/CIRCRESAHA.117.311819. PMID: 29021296. PMCID: PMC5681426. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85037676960&origin=inward.
Article15. Gomez-Cabrera MC, Domenech E, Viña J. 2008; Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 44:126–131. DOI: 10.1016/j.freeradbiomed.2007.02.001. PMID: 18191748. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=37849021505&origin=inward.
Article16. Griffiths-Jones S. 2004; The microRNA Registry. Nucleic Acids Res. 32:D109–D111. DOI: 10.1093/nar/gkh023. PMID: 14681370. PMCID: PMC308757.
Article17. Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. 2006; miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34:D140–D144. DOI: 10.1093/nar/gkj112. PMID: 16381832. PMCID: PMC1347474. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33644750115&origin=inward.
Article18. Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, inivasan A Sr, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. 2000; Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 408:86–89. DOI: 10.1038/35040556. PMID: 11081512. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034597777&origin=inward.
Article19. Yang F, Chen Q, He S, Yang M, Maguire EM, An W, Afzal TA, Luong LA, Zhang L, Xiao Q. 2018; miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation. 137:1824–1841. DOI: 10.1161/CIRCULATIONAHA.117.027799. PMID: 29246895. PMCID: PMC5916488. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85051770266&origin=inward.
Article20. Tang Y, Yu S, Liu Y, Zhang J, Han L, Xu Z. 2017; MicroRNA-124 controls human vascular smooth muscle cell phenotypic switch via Sp1. Am J Physiol Heart Circ Physiol. 313:H641–H649. DOI: 10.1152/ajpheart.00660.2016. PMID: 28667053. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029370012&origin=inward.
Article21. Iaconetti C, De Rosa S, Polimeni A, Sorrentino S, Gareri C, Carino A, Sabatino J, Colangelo M, Curcio A, Indolfi C. 2015; Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo. Cardiovasc Res. 107:522–533. DOI: 10.1093/cvr/cvv141. PMID: 25994172. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84948129366&origin=inward.
Article22. Yue Y, Zhang Z, Zhang L, Chen S, Guo Y, Hong Y. 2018; miR-143 and miR-145 promote hypoxia-induced proliferation and migration of pulmonary arterial smooth muscle cells through regulating ABCA1 expression. Cardiovasc Pathol. 37:15–25. DOI: 10.1016/j.carpath.2018.08.003. PMID: 30195228. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052849462&origin=inward.
Article23. Grundmann S, Hans FP, Kinniry S, Heinke J, Helbing T, Bluhm F, Sluijter JP, Hoefer I, Pasterkamp G, Bode C, Moser M. 2011; MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 123:999–1009. DOI: 10.1161/CIRCULATIONAHA.110.000323. PMID: 21339483. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79952739991&origin=inward.
Article24. Torella D, Iaconetti C, Catalucci D, Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila I, Curcio A, Condorelli G, Indolfi C. 2011; MicroRNA-133 controls vascular smooth muscle cell phenotypic switch in vitro and vascular remodeling in vivo. Circ Res. 109:880–893. DOI: 10.1161/CIRCRESAHA.111.240150. PMID: 21852550.
Article25. Luo Y, Xiong W, Dong S, Liu F, Liu H, Li J. 2017; MicroRNA-146a promotes the proliferation of rat vascular smooth muscle cells by downregulating p53 signaling. Mol Med Rep. 16:6940–6945. DOI: 10.3892/mmr.2017.7477. PMID: 28901447. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030107419&origin=inward.
Article26. Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. 2009; A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 104:476–487. DOI: 10.1161/CIRCRESAHA.108.185363. PMID: 19150885. PMCID: PMC2728290. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=61949252089&origin=inward.
Article27. Merlet E, Atassi F, Motiani RK, Mougenot N, Jacquet A, Nadaud S, Capiod T, Trebak M, Lompré AM, Marchand A. 2013; miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res. 98:458–468. DOI: 10.1093/cvr/cvt045. PMID: 23447642. PMCID: PMC3656613. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84877949558&origin=inward.
Article28. Tang R, Mei X, Wang YC, Cui XB, Zhang G, Li W, Chen SY. 2019; LncRNA GAS5 regulates vascular smooth muscle cell cycle arrest and apoptosis via p53 pathway. Biochim Biophys Acta Mol Basis Dis. 1865:2516–2525. DOI: 10.1016/j.bbadis.2019.05.022. PMID: 31167125. PMCID: PMC6663079. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067497328&origin=inward.
Article29. Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D'Acquisto F, Bland EJ, Bombardieri M, Pitzalis C, Perretti M, Marelli-Berg FM, Mauro C. 2015; Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13:e1002202. DOI: 10.1371/journal.pbio.1002202. PMID: 26181372. PMCID: PMC4504715. PMID: 23101ff17da749d2a6752ee7354ed8ab. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84938702403&origin=inward.
Article30. Butler TM, Siegman MJ. 1985; High-energy phosphate metabolism in vascular smooth muscle. Annu Rev Physiol. 47:629–643. DOI: 10.1146/annurev.ph.47.030185.003213. PMID: 3158271.
Article31. Paul RJ. 1983; Functional compartmentalization of oxidative and glycolytic metabolism in vascular smooth muscle. Am J Physiol. 244:C399–409. DOI: 10.1152/ajpcell.1983.244.5.C399. PMID: 6846528. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0020582997&origin=inward.
Article32. Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. 2013; Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 88:1127–1140. DOI: 10.1016/j.mayocp.2013.06.012. PMID: 24079682. PMCID: PMC3975915. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84888627079&origin=inward.
Article33. Leite TC, Coelho RG, Da Silva D, Coelho WS, Marinho-Carvalho MM, Sola-Penna M. 2011; Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Lett. 585:92–98. DOI: 10.1016/j.febslet.2010.11.009. PMID: 21074528. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78650911575&origin=inward.
Article34. Zhao LL, Wu H, Sun JL, Liao L, Cui C, Liu Q, Luo J, Tang XH, Luo W, Ma JD, Ye X, Li SJ, Yang S. 2020; MicroRNA-124 regulates lactate transportation in the muscle of largemouth bass (micropterus salmoides) under hypoxia by targeting MCT1. Aquat Toxicol. 218:105359. DOI: 10.1016/j.aquatox.2019.105359. PMID: 31765944. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075308374&origin=inward.
Article35. Ping W, Senyan H, Li G, Yan C, Long L. 2018; Increased lactate in gastric cancer tumor-infiltrating lymphocytes is related to impaired T cell function due to miR-34a deregulated lactate dehydrogenase A. Cell Physiol Biochem. 49:828–836. DOI: 10.1159/000493110. PMID: 30165351. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85052941175&origin=inward.
Article36. Bang C, Fiedler J, Thum T. 2012; Cardiovascular importance of the microRNA-23/27/24 family. Microcirculation. 19:208–214. DOI: 10.1111/j.1549-8719.2011.00153.x. PMID: 22136461. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84859125017&origin=inward.
Article37. Majid S, Dar AA, Saini S, Arora S, Shahryari V, Zaman MS, Chang I, Yamamura S, Tanaka Y, Deng G, Dahiya R. 2012; miR-23b represses proto-oncogene Src kinase and functions as methylation-silenced tumor suppressor with diagnostic and prognostic significance in prostate cancer. Cancer Res. 72:6435–6446. DOI: 10.1158/0008-5472.CAN-12-2181. PMID: 23074286. PMCID: PMC3940348. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84871222985&origin=inward.
Article38. Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. 2012; The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med. 18:1077–1086. DOI: 10.1038/nm.2815. PMID: 22660635. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84863686039&origin=inward.
Article39. Plekhanova OS, Parfyonova YV, Bibilashvily RSh, Stepanova VV, Erne P, Bobik A, Tkachuk VA. 2000; Urokinase plasminogen activator enhances neointima growth and reduces lumen size in injured carotid arteries. J Hypertens. 18:1065–1069. DOI: 10.1097/00004872-200018080-00011. PMID: 10953998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033851467&origin=inward.
Article40. Clowes AW, Clowes MM, Au YP, Reidy MA, Belin D. 1990; Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 67:61–67. DOI: 10.1161/01.RES.67.1.61. PMID: 2114227. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0025295728&origin=inward.
Article41. Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. 2009; Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc Res. 84:326–335. DOI: 10.1093/cvr/cvp220. PMID: 19570811. PMCID: PMC2761202. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=71549123218&origin=inward.
Article42. Tsai S, Hollenbeck ST, Ryer EJ, Edlin R, Yamanouchi D, Kundi R, Wang C, Liu B, Kent KC. 2009; TGF-beta through Smad3 signaling stimulates vascular smooth muscle cell proliferation and neointimal formation. Am J Physiol Heart Circ Physiol. 297:H540–H549. DOI: 10.1152/ajpheart.91478.2007. PMID: 19525370. PMCID: PMC2724222. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=68049103323&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- PLK2 Single Nucleotide Variant in Gastric Cancer Patients Affects miR-23b-5p Binding
- rBMSCs/ITGA5B1 Promotes Human Vascular Smooth Muscle Cell Differentiation via Enhancing Nitric Oxide Production
- Improved Differentiation Ability and Therapeutic Effect of miR-23a-3p Expressing Bone Marrow-Derived Mesenchymal Stem Cells in Mice Model with Acute Lung Injury
- Activating transcription factor 4 aggravates angiotensin IIinduced cell dysfunction in human vascular aortic smooth muscle cells via transcriptionally activating fibroblast growth factor 21
- The Contribution of Resident Vascular Stem Cells to Arterial Pathology