Cancer Res Treat.
2022 Oct;54(4):1111-1120. 10.4143/crt.2021.1017.
The Association of Estrogen Receptor Activity, Interferon Signaling, and MHC Class I Expression in Breast Cancer
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2NeogenTC Corp., Seoul, Korea
- 3Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2534190
- DOI: http://doi.org/10.4143/crt.2021.1017
Abstract
- Purpose
The expression of major histocompatibility complex class I (MHC I) has previously been reported to be negatively associated with estrogen receptor (ER) expression. Furthermore, MHC I expression, level of tumor-infiltrating lymphocytes (TILs), and expression of interferon (IFN) mediator MxA are positively associated with one another in human breast cancers. This study aimed to investigate the mechanisms of association of MHC I with ER and IFN signaling.
Materials and Methods
The human leukocyte antigen (HLA)-ABC protein expression was analyzed in breast cancer cell lines. The expressions of HLA-A and MxA mRNAs were analyzed in MCF-7 cells in Gene Expression Omnibus (GEO) data. ER and HLA-ABC expressions, Ki-67 labeling index and TIL levels in tumor tissue were also analyzed in ER+/ human epidermal growth factor receptor 2 (HER2)- breast cancer patients who randomly received either neoadjuvant chemotherapy or estrogen modulator treatment followed by resection.
Results
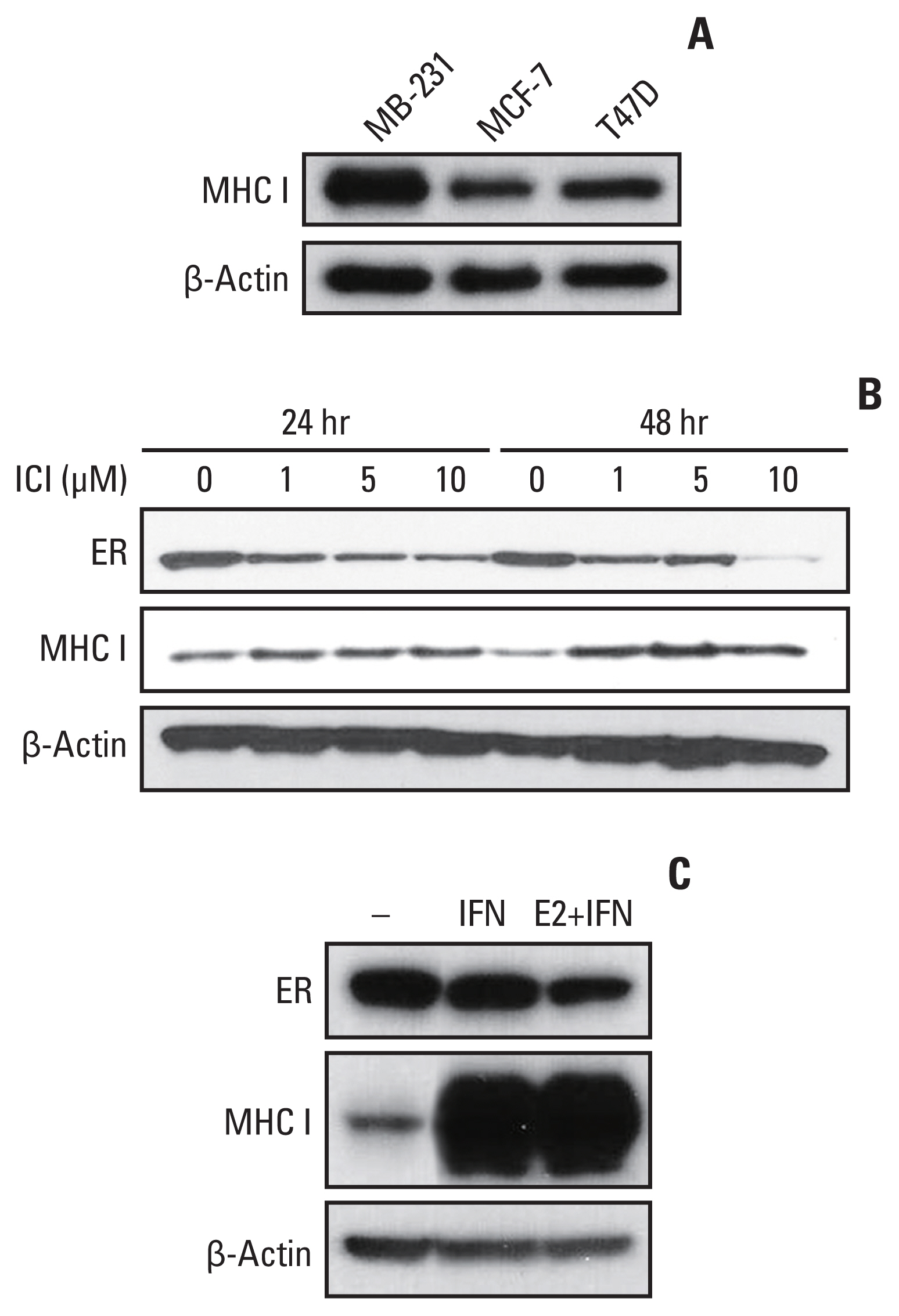
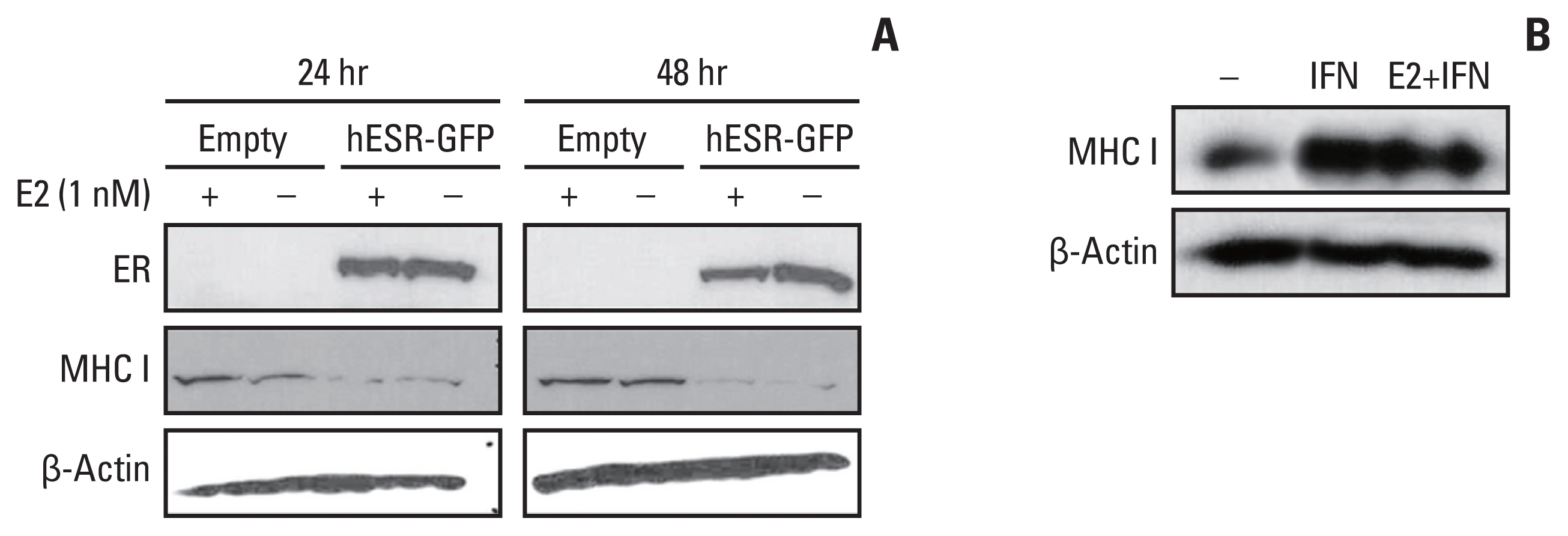
HLA-ABC protein expression was decreased after β-estradiol treatment or hESR-GFP transfection and increased after fulvestrant or IFN-γ treatment in cell lines. In GEO data, HLA-A and MxA expression was increased after ESR1 shRNA transfection. In patients, ER Allred score was significantly lower and the HLA-ABC expression, TIL levels, and Ki-67 were significantly higher in the estrogen modulator treated group than the chemotherapy treated group.
Conclusion
MHC I expression and TIL levels might be affected by ER pathway modulation and IFN treatment. Further studies elucidating the mechanism of MHC I regulation could suggest a way to boost TIL influx in cancer in a clinical setting.
Keyword
Figure
Reference
-
References
1. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013; 31:860–7.
Article2. Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010; 28:105–13.
Article3. Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013; 16:32–9.
Article4. Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, et al. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013; 109:2705–13.
Article5. Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014; 32:2959–66.
Article6. Phan GQ, Rosenberg SA. Adoptive cell transfer for patients with metastatic melanoma: the potential and promise of cancer immunotherapy. Cancer Control. 2013; 20:289–97.
Article7. Agrawal S, Kishore MC. MHC class I gene expression and regulation. J Hematother Stem Cell Res. 2000; 9:795–812.
Article8. Torigoe T, Asanuma H, Nakazawa E, Tamura Y, Hirohashi Y, Yamamoto E, et al. Establishment of a monoclonal anti-pan HLA class I antibody suitable for immunostaining of formalin-fixed tissue: unusually high frequency of down-regulation in breast cancer tissues. Pathol Int. 2012; 62:303–8.
Article9. Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014; 20:1301–9.
Article10. Lee HJ, Song IH, Park IA, Heo SH, Kim YA, Ahn JH, et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget. 2016; 7:30119–32.
Article11. Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn JH, et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. 2015; 144:278–88.
Article12. Kim YA, Lee HJ, Heo SH, Park HS, Park SY, Bang W, et al. MxA expression is associated with tumor-infiltrating lymphocytes and is a prognostic factor in triple-negative breast cancer. Breast Cancer Res Treat. 2016; 156:597–606.
Article13. Al Saleh S, Al Mulla F, Luqmani YA. Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells. PLoS One. 2011; 6:e20610.
Article14. Kim HJ, Noh WC, Lee ES, Jung YS, Kim LS, Han W, et al. Efficacy of neoadjuvant endocrine therapy compared with neoadjuvant chemotherapy in pre-menopausal patients with oestrogen receptor-positive and HER2-negative, lymph node-positive breast cancer. Breast Cancer Res. 2020; 22:54.
Article15. Allison KH, Brogi E, Ellis IO, Fox SB, Morris EA, Sahin A, et al. WHO classification of tumours: breast tumours. 5th ed. Lyon: IARC Press;2019.16. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–71.
Article17. Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003; 12:320–7.
Article18. Formenti SC, Spicer D, Skinner K, Cohen D, Groshen S, Bettini A, et al. Low HER2/neu gene expression is associated with pathological response to concurrent paclitaxel and radiation therapy in locally advanced breast cancer. Int J Radiat Oncol Biol Phys. 2002; 52:397–405.
Article19. R Core Team. R: a language and environment for statistical computing. 323 ed. Vienna: R Foundation for Statistical Computing;2015.20. Song IH, Heo SH, Bang WS, Park HS, Park IA, Kim YA, et al. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat. 2017; 49:399–407.
Article21. Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015; 3:326–32.
Article22. Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016; 4:59.
Article23. Berger ER, Park T, Saridakis A, Golshan M, Greenup RA, Ahuja N. Immunotherapy treatment for triple negative breast cancer. Pharmaceuticals (Basel). 2021; 14:763.
Article24. Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A. 2016; 113:E7759–68.
Article25. Mostafa AA, Codner D, Hirasawa K, Komatsu Y, Young MN, Steimle V, et al. Activation of ERalpha signaling differentially modulates IFN-gamma induced HLA-class II expression in breast cancer cells. PLoS One. 2014; 9:e87377.26. Anandappa AJ, Wu CJ, Ott PA. Directing traffic: how to effectively drive T cells into tumors. Cancer Discov. 2020; 10:185–97.
Article27. Castaneda CA, Mittendorf E, Casavilca S, Wu Y, Castillo M, Arboleda P, et al. Tumor infiltrating lymphocytes in triple negative breast cancer receiving neoadjuvant chemotherapy. World J Clin Oncol. 2016; 7:387–94.
Article28. Park YH, Lal S, Lee JE, Choi YL, Wen J, Ram S, et al. Chemotherapy induces dynamic immune responses in breast cancers that impact treatment outcome. Nat Commun. 2020; 11:6175.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcription of class II MHC gene by interferon-gamma in FRTL-5 cells
- Partial purification and characterization of a novel murine factor that augments the expression of class I MHC antigens on tumor cells
- Expression of Cyclooxygenase-2 in Human Breast Carcinoma: Relevance to Tumor Angiogenesis and Expression of Estrogen Receptor
- Monophosphoryl lipid A (MPL) upregulates major histocompatibility complex (MHC) class I expression by increasing interferon-gamma (IFN-gamma)
- Role of Estrogen Receptor-alpha in the Regulation of Claudin-6 Expression in Breast Cancer Cells