Cancer Res Treat.
2022 Jul;54(3):917-925. 10.4143/crt.2021.399.
Outcomes of Anti-CD19 CAR-T Treatment of Pediatric B-ALL with Bone Marrow and Extramedullary Relapse
- Affiliations
-
- 1Department of Hematology and Oncology, Key laboratory of Pediatric Hematology and Oncology Ministry of Health, Shanghai Children's Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Hematology/Oncology, Children's Hospital of Soochow University, Jiangsu, China
- KMID: 2531337
- DOI: http://doi.org/10.4143/crt.2021.399
Abstract
- Purpose
Anti-CD19 chimeric antigen receptor T-cell immunotherapy (19CAR-T) has achieved impressive clinical results in adult and pediatric relapsed/refractory (r/r) B-lineage acute lymphoblastic leukemia (B-ALL). However, the application and effect of CAR-T therapy in B-ALL patients with extramedullary relapse are rarely issued even disqualified in some clinical trials. Here, we examined the efficacy of 19CAR-T in patients with both bone marrow and extramedullary involvement.
Materials and Methods
CAR-T cells were generated by transfection of primary human T lymphocytes with a lentiviral vector expressing anti-CD19 single chain antibody fragments (scFvs) with the cytoplasmic domains of 4-1BB and CD3ζ, and used to infuse patients diagnosed as having r/r B-ALL with extramedullary origination. Clinical responses were evaluated by the use of bone marrow aspiration, imaging, and flow cytometry.
Results
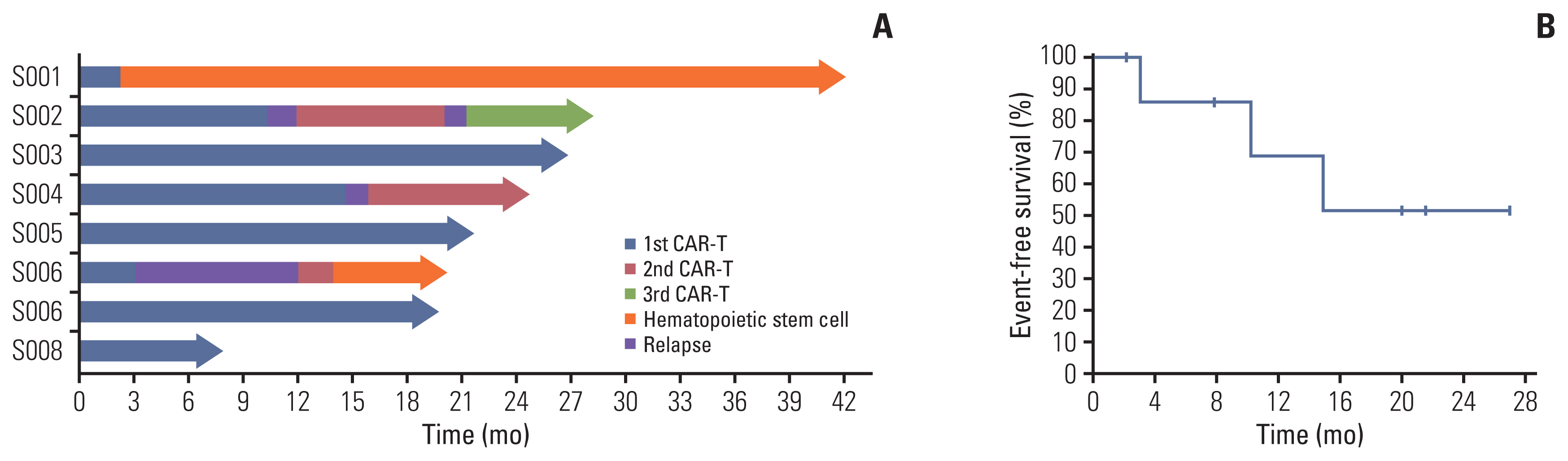
Eight patients received 19CAR-T infusion and all attained complete remission (CR). Only one patient was bridged to hematopoietic stem cell transplantation (HSCT). Although three patients relapsed after infusion, they received 19/22CAR-T infusion sequentially and attained a second remission. To date, five patients are in continuous CR and all eight patients are still alive. The mean follow-up time was 21.9 months, while the 24-month estimated event-free survival is 51.4%.
Conclusion
19CAR-T therapy can lead to clinical remission for extramedullary relapsed pediatric B-ALL patients. However, the problem of CD19+ relapses after CAR-T remained to be solved. For patients relapsing after CAR-T, a second CAR-T therapy creates another opportunity for remission for subsequent HSCT.
Keyword
Figure
Reference
-
References
1. Heikamp EB, Pui CH. Next-generation evaluation and treatment of pediatric acute lymphoblastic leukemia. J Pediatr. 2018; 203:14–24.
Article2. Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015; 33:2938–48.
Article3. Ding LW, Sun QY, Mayakonda A, Tan KT, Chien W, Lin DC, et al. Mutational profiling of acute lymphoblastic leukemia with testicular relapse. J Hematol Oncol. 2017; 10:65.
Article4. Kosucu P, Kul S, Gunes G, Yilmaz M, Ersoz S, Ozdemir F. Multiple relapses in extramedullary localization of acute lymphoblastic leukemia. Bratisl Lek Listy. 2012; 113:46–9.
Article5. Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013; 14:e205–17.
Article6. Kondoh T, Kuribayashi K, Tanaka M, Kobayashi D, Yanagihara N, Watanabe N. CD7 promotes extramedullary involvement of the B-cell acute lymphoblastic leukemia line Tanoue by enhancing integrin beta2-dependent cell adhesiveness. Int J Oncol. 2014; 45:1073–81.7. Sun W, Malvar J, Sposto R, Verma A, Wilkes JJ, Dennis R, et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia. 2018; 32:2316–25.
Article8. von Stackelberg A, Volzke E, Kuhl JS, Seeger K, Schrauder A, Escherich G, et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. Eur J Cancer. 2011; 47:90–7.
Article9. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989; 86:10024–8.
Article10. Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol. 2015; 36:494–502.
Article11. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018; 378:439–48.
Article12. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017; 129:3322–31.
Article13. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015; 385:517–28.
Article14. Curran KJ, Margossian SP, Kernan NA, Silverman LB, Williams DA, Shukla N, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019; 134:2361–8.
Article15. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019; 25:625–38.
Article16. Weng J, Lai P, Qin L, Lai Y, Jiang Z, Luo C, et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J Hematol Oncol. 2018; 11:25.
Article17. Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013; 50:185–96.
Article18. Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017; 376:836–47.
Article19. Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O’Donnell M, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017; 92:858–65.
Article20. Jabbour E, Dull J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018; 93:371–4.
Article21. Chen X, Wang Y, Ruan M, Li J, Zhong M, Li Z, et al. Treatment of testicular relapse of B-cell acute lymphoblastic leukemia with CD19-specific chimeric antigen receptor T cells. Clin Lymphoma Myeloma Leuk. 2020; 20:366–70.
Article22. Talekar MK, Maude SL, Hucks GE, Motley LS, Callahan C, White CM, et al. Effect of chimeric antigen receptor-modified T (CAR-T) cells on responses in children with non-CNS extramedullary relapse of CD19+ acute lymphoblastic leukemia (ALL). J Clin Oncol. 2017; 35(15 Suppl):10507.
Article23. Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015; 4:e1027469.
Article24. Jacoby E, Bielorai B, Avigdor A, Itzhaki O, Hutt D, Nussboim V, et al. Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. Am J Hematol. 2018; 93:1485–92.
Article25. Schafer H, Bader P, Kaiserling E, Klingebiel T, Handgretinger R, Kanz L, et al. Extramedullary relapses at uncommon sites after allogeneic stem cell transplantation. Bone Marrow Transplant. 2000; 26:1133–5.
Article26. Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018; 8:958–71.
Article27. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017; 7:1404–19.
Article28. Lee DW, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C, Yates B, et al. Long-term outcomes following CD19 CAR T cell therapy for B-ALL are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Blood. 2016; 128:218.
Article29. Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018; 378:449–59.
Article30. Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019; 133:1652–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolated Extramedullary Relapse of Acute Lymphoblastic Leukemia Presenting as an Paraspinal Mass
- Long-Term Complete Remission in an Acute Myeloid Leukemia Patient with Isolated Central Nervous System Relapse after Allogeneic Hematopoietic Stem Cell Transplantation
- An Unusual Relapse of Acute Lymphoblastic Leukemia in the Uterine Corpus
- A Case of Extramedullary Relapse of Acute Promyelocytic Leukemia in the External Auditory Canal and Temporal Bone
- Proportions of Cells Expressing CD38-/CD34+, CD38+/CD34+, CD19+/CD34+, or CD13,33+/CD34+ in the Regenerating Bone Marrows During Complete Remission of Acute Leukemia or After Bone Marrow Transplantation