Cancer Res Treat.
2022 Jul;54(3):767-781. 10.4143/crt.2021.651.
Integrin αvβ3 Induces HSP90 Inhibitor Resistance via FAK Activation in KRAS-Mutant Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea
- 3Department of Biomedical Sciences, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Bio & Drug Discovery Division, Center for Drug Discovery Technology, Korea Research Institute of Chemical Technology, Daejeon, Korea
- 6Department of Orthopaedic Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Center for Advancing Cancer Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 8Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2531323
- DOI: http://doi.org/10.4143/crt.2021.651
Abstract
- Purpose
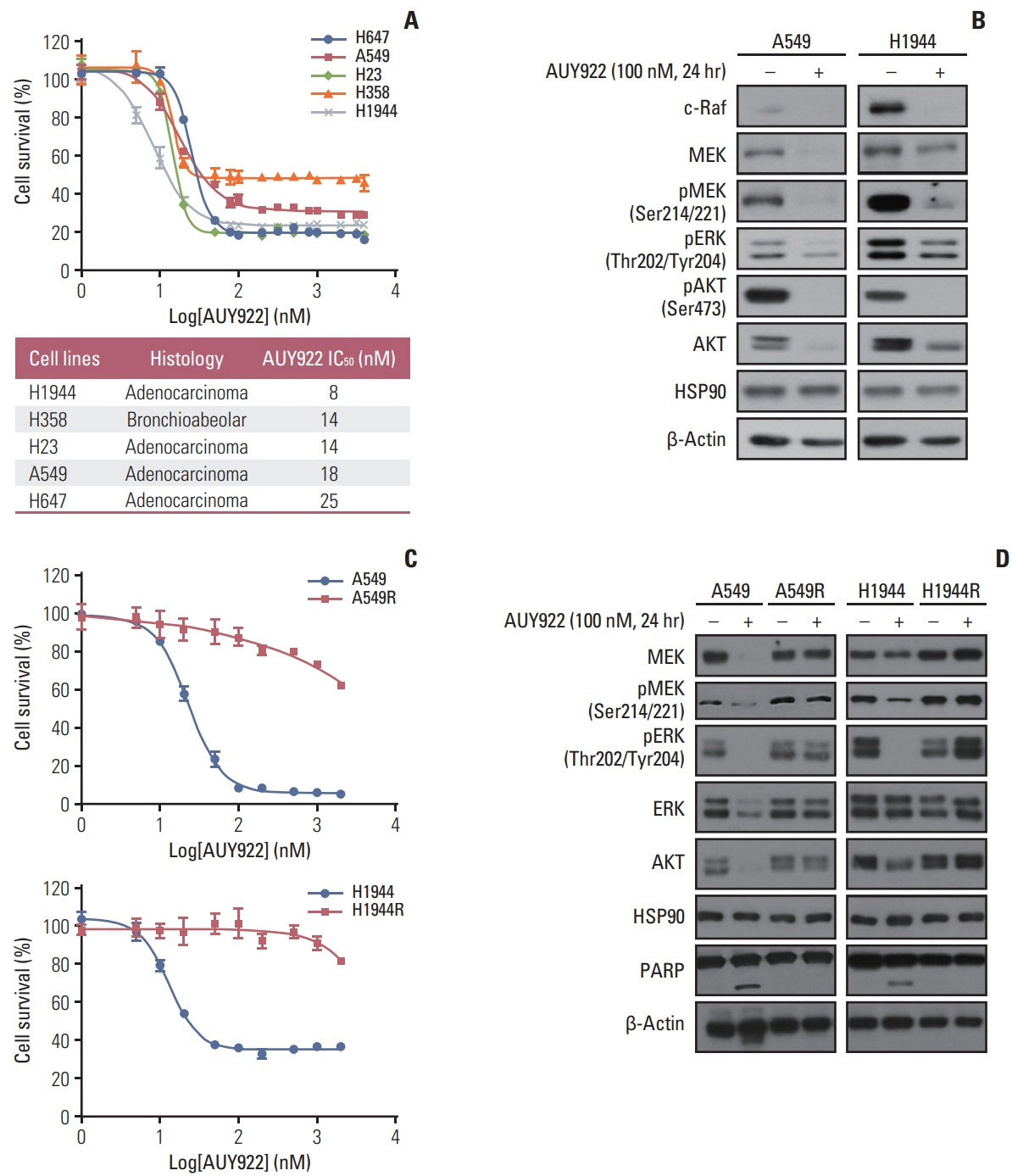
Heat shock protein-90 (HSP90) remains an important cancer target because of its involvement in multiple oncogenic protein pathways and biologic processes. Although many HSP90 inhibitors have been tested in the treatment of KRAS-mutant non–small cell lung cancer (NSCLC), most, including AUY922, have failed due to toxic effects and resistance generation, even though a modest efficacy has been observed for these drugs in clinical trials. In our present study, we investigated the novel mechanism of resistance to AUY922 to explore possible avenues of overcoming and want to provide some insights that may assist with the future development of successful next-generation HSP90 inhibitors.
Materials and Methods
We established two AUY922-resistant KRAS-mutated NSCLC cells and conducted RNA sequencing to identify novel resistance biomarker.
Results
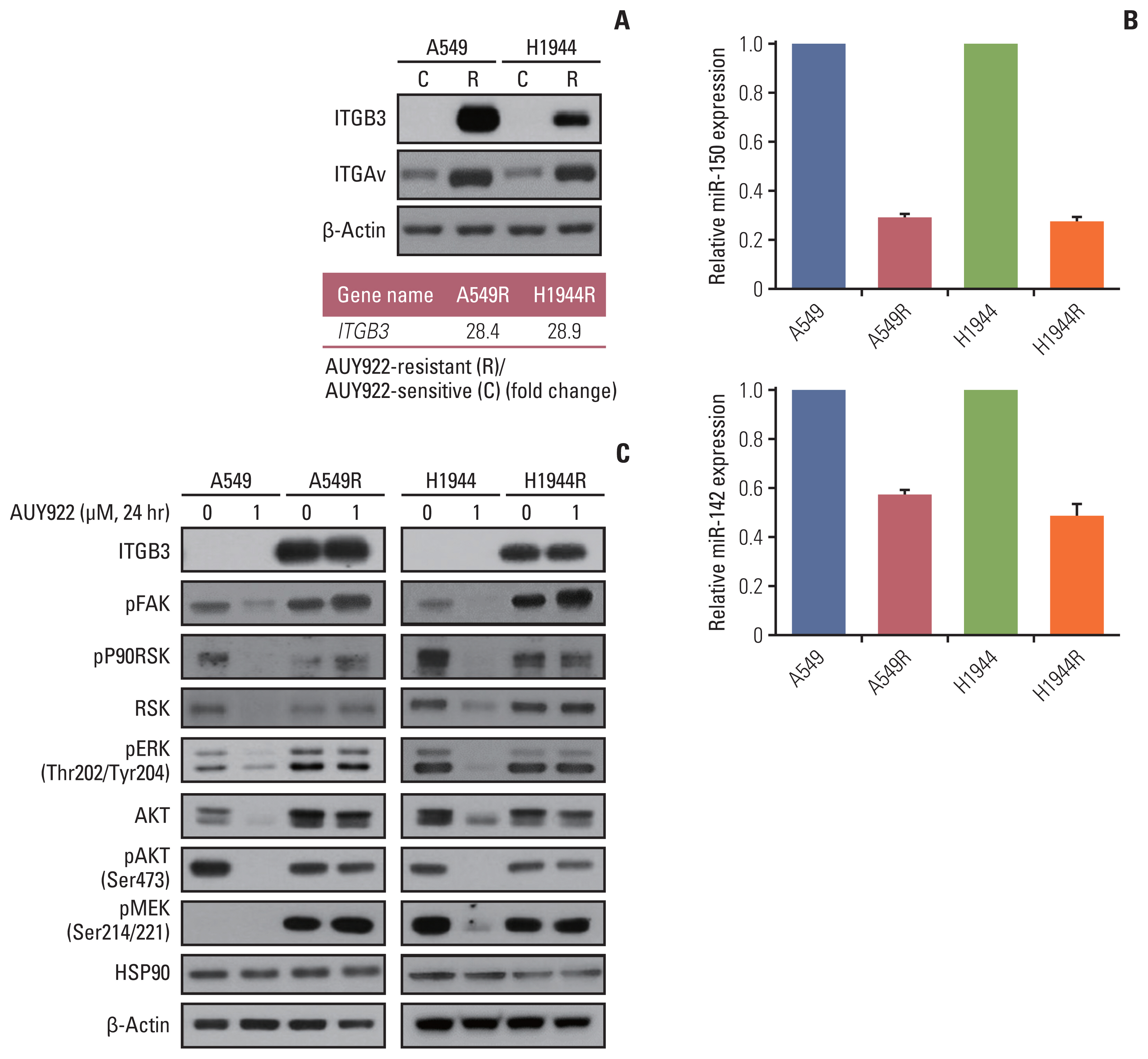
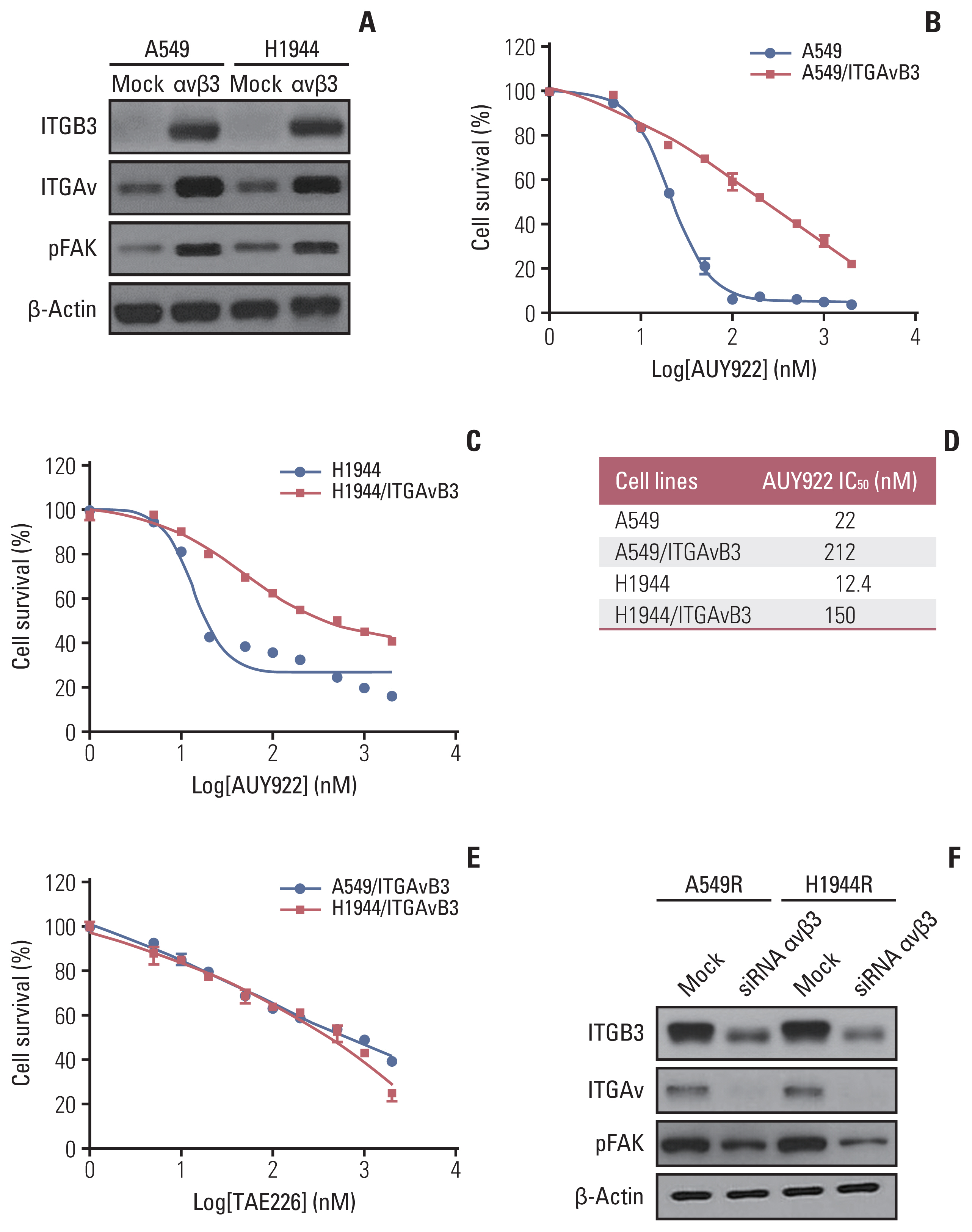
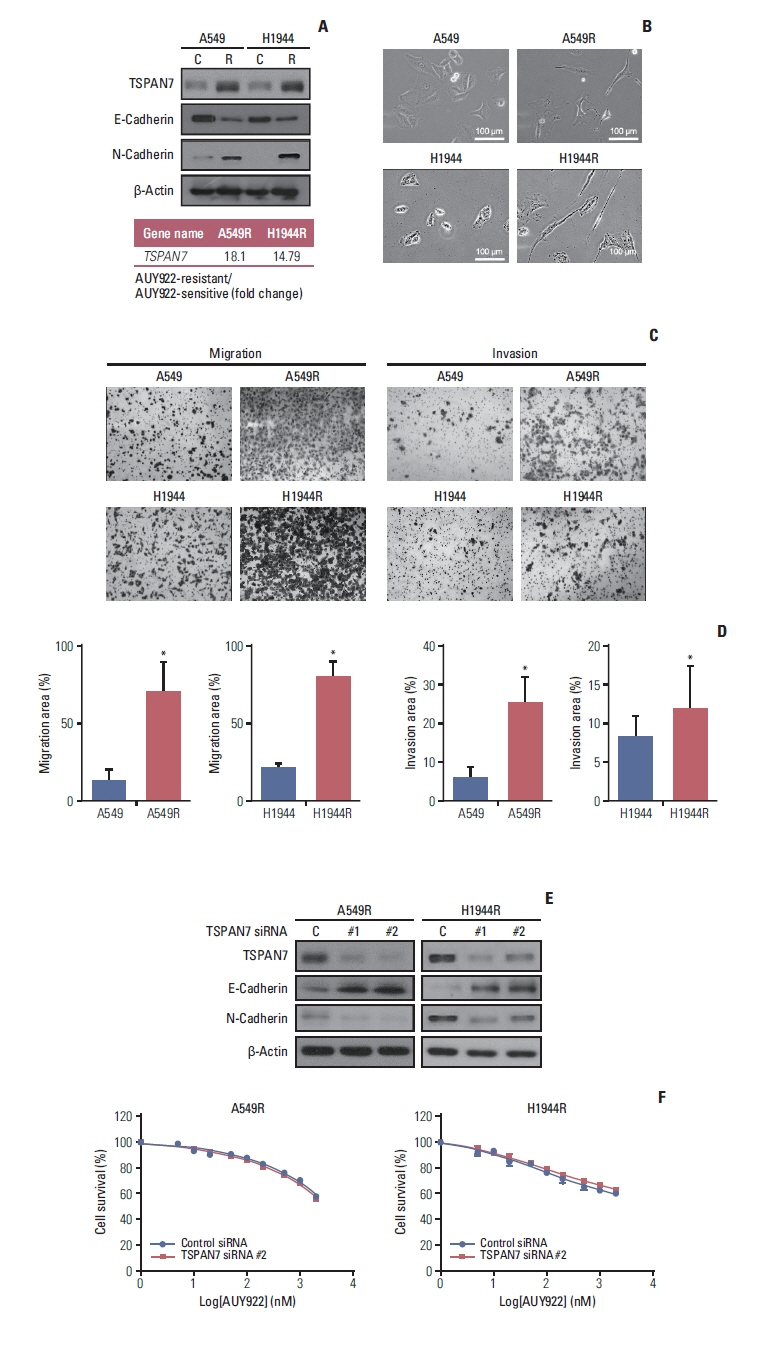
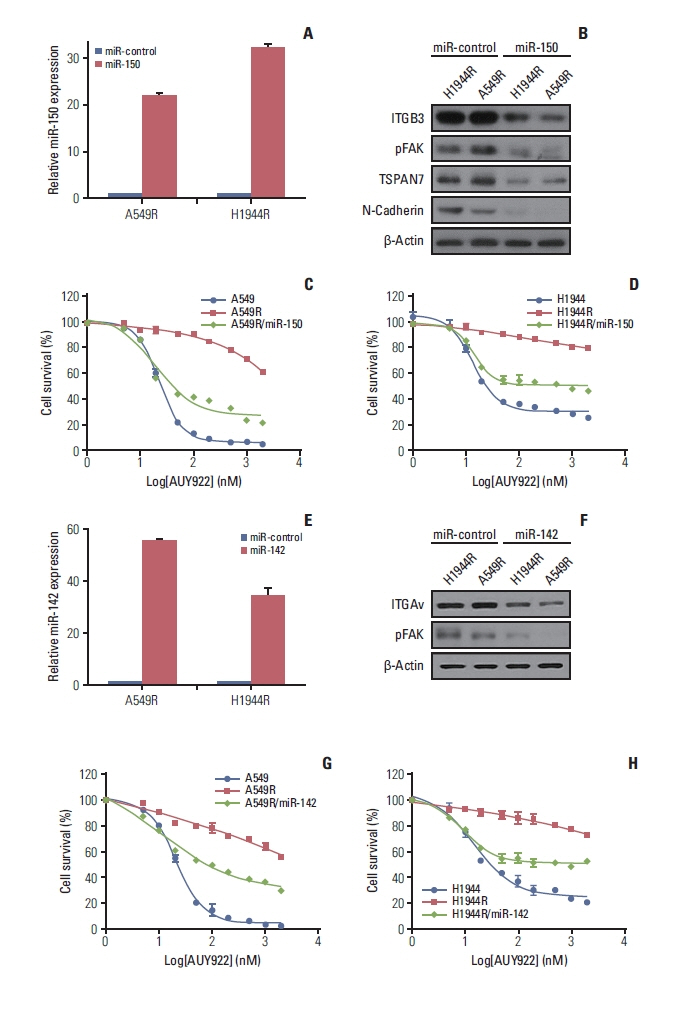
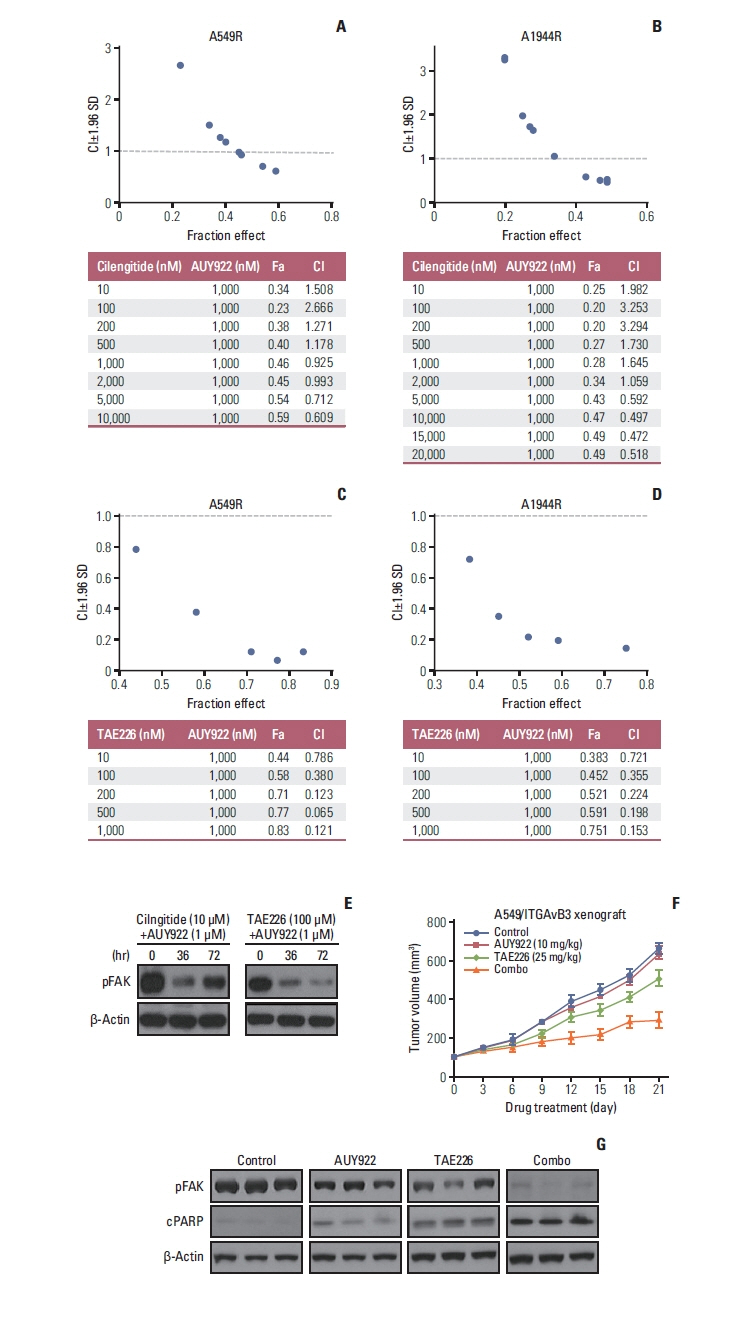
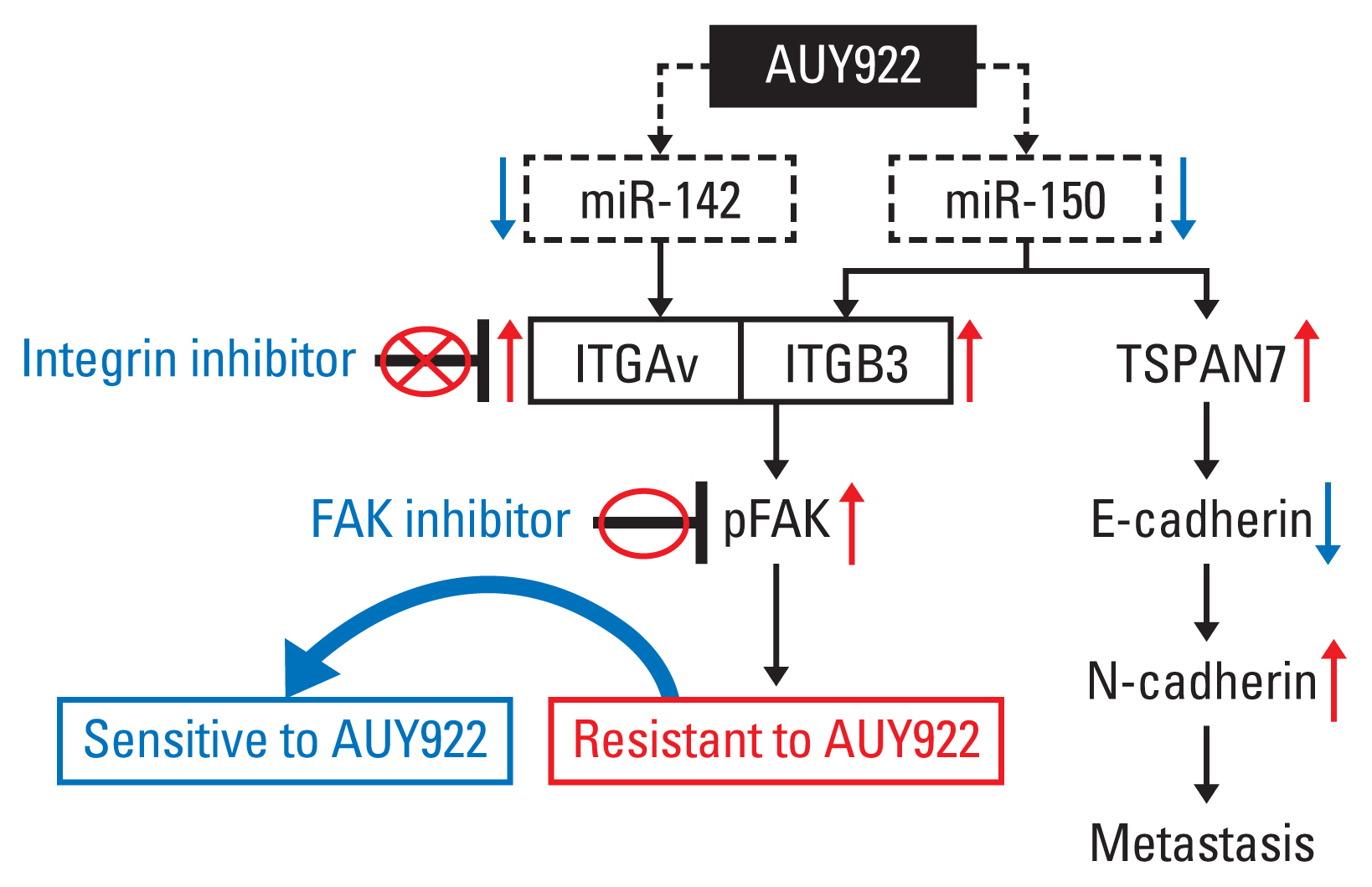
We identified novel two resistance biomarkers. We observed that both integrin Av (ITGAv) and β3 (ITGB3) induce AUY922-resistance via focal adhesion kinase (FAK) activation, as well as an epithelial-mesenchymal transition, in both in vitro and in vivo xenograft model. mRNAs of both ITGAv and ITGB3 were also found to be elevated in a patient who had shown acquired resistance in a clinical trial of AUY922. ITGAv was induced by miR-142 downregulation, and ITGB3 was increased by miR-150 downregulation during the development of AUY922-resistance. Therefore, miR-150 and miR-142 overexpression effectively inhibited ITGAvB3-dependent FAK activation, restoring sensitivity to AUY922.
Conclusion
The synergistic co-targeting of FAK and HSP90 attenuated the growth of ITGAvB3-induced AUY922-resistant KRAS-mutated NSCLC cells in vitro and in vivo, suggesting that this combination may overcome acquired AUY922-resistance in KRAS-mutant NSCLC.
Keyword
Figure
Reference
-
References
1. Berndt N, Hamilton AD, Sebti SM. Targeting protein prenylation for cancer therapy. Nat Rev Cancer. 2011; 11:775–91.
Article2. Park KS, Oh B, Lee MH, Nam KY, Jin HR, Yang H, et al. The HSP90 inhibitor, NVP-AUY922, sensitizes KRAS-mutant non-small cell lung cancer with intrinsic resistance to MEK inhibitor, trametinib. Cancer Lett. 2016; 372:75–81.
Article3. Park KS, Yang H, Choi J, Seo S, Kim D, Lee CH, et al. The HSP90 inhibitor, NVP-AUY922, attenuates intrinsic PI3K inhibitor resistance in KRAS-mutant non-small cell lung cancer. Cancer Lett. 2017; 406:47–53.
Article4. Papke B, Der CJ. Drugging RAS: know the enemy. Science. 2017; 355:1158–63.
Article5. Ostrem JM, Shokat KM. Direct small-molecule inhibitors of KRAS: from structural insights to mechanism-based design. Nat Rev Drug Discov. 2016; 15:771–85.
Article6. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020; 383:1207–17.
Article7. Janne PA, Rybkin II, Spira AI, Riely GJ, Papadopoulos KP, Sabari JK, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in advanced/metastatic non-small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur J Cancer. 2020; 138(Suppl 2):S1–2.8. Johnson ML, Ou SH, Barve M, Rybkin II, Papadopoulos KP, Leal TA, et al. KRYSTAL-1: activity and safety of adagrasib (MRTX849) in patients with colorectal cancer (CRC) and other solid tumors harboring a KRAS G12C mutation. Eur J Cancer. 2020; 138(Suppl 2):S2.
Article9. Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013; 14:e358–69.
Article10. Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci U S A. 1994; 91:8324–8.
Article11. Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, et al. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998; 3:100–8.
Article12. Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005; 5:761–72.
Article13. Neckers L, Blagg B, Haystead T, Trepel JB, Whitesell L, Picard D. Methods to validate Hsp90 inhibitor specificity, to identify off-target effects, and to rethink approaches for further clinical development. Cell Stress Chaperones. 2018; 23:467–82.
Article14. Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov. 2014; 9:1–20.
Article15. Seggewiss-Bernhardt R, Bargou RC, Goh YT, Stewart AK, Spencer A, Alegre A, et al. Phase 1/1B trial of the heat shock protein 90 inhibitor NVP-AUY922 as monotherapy or in combination with bortezomib in patients with relapsed or refractory multiple myeloma. Cancer. 2015; 121:2185–92.
Article16. Ambros V. The functions of animal microRNAs. Nature. 2004; 431:350–5.
Article17. Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther. 2010; 17:523–31.
Article18. Schwickert A, Weghake E, Bruggemann K, Engbers A, Brinkmann BF, Kemper B, et al. microRNA miR-142-3p inhibits breast cancer cell invasiveness by synchronous targeting of WASL, integrin alpha V, and additional cytoskeletal elements. PLoS One. 2015; 10:e0143993.
Article19. Borschel CS, Stejskalova A, Schafer SD, Kiesel L, Gotte M. miR-142-3p reduces the size, migration, and contractility of endometrial and endometriotic stromal cells by targeting integrin- and Rho GTPase-related pathways that regulate cytoskeletal function. Biomedicines. 2020; 8:291.
Article20. Honda N, Jinnin M, Kira-Etoh T, Makino K, Kajihara I, Makino T, et al. miR-150 down-regulation contributes to the constitutive type I collagen overexpression in scleroderma dermal fibroblasts via the induction of integrin beta3. Am J Pathol. 2013; 182:206–16.21. Guan JL. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 2010; 62:268–76.
Article22. Alanko J, Ivaska J. Endosomes: emerging platforms for integrin-mediated FAK signalling. Trends Cell Biol. 2016; 26:391–8.
Article23. Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006; 18:516–23.
Article24. Wang X, Lin M, Zhao J, Zhu S, Xu M, Zhou X. TSPAN7 promotes the migration and proliferation of lung cancer cells via epithelial-to-mesenchymal transition. Onco Targets Ther. 2018; 11:8815–22.
Article25. Chen W, Harbeck MC, Zhang W, Jacobson JR. MicroRNA regulation of integrins. Transl Res. 2013; 162:133–43.
Article26. Busacca S, Law EW, Powley IR, Proia DA, Sequeira M, Le Quesne J, et al. Resistance to HSP90 inhibition involving loss of MCL1 addiction. Oncogene. 2016; 35:1483–92.
Article27. Mumin NH, Drobnitzky N, Patel A, Lourenco LM, Cahill FF, Jiang Y, et al. Overcoming acquired resistance to HSP90 inhibition by targeting JAK-STAT signalling in triple-negative breast cancer. BMC Cancer. 2019; 19:102.
Article28. Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003; 4:123–31.29. Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004; 5:816–26.
Article30. Lu J, Guo S, Ebert BL, Zhang H, Peng X, Bosco J, et al. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev Cell. 2008; 14:843–53.
Article31. Suetsugu T, Koshizuka K, Seki N, Mizuno K, Okato A, Arai T, et al. Downregulation of matrix metalloproteinase 14 by the antitumor miRNA, miR-150-5p, inhibits the aggressiveness of lung squamous cell carcinoma cells. Int J Oncol. 2018; 52:913–24.
Article32. Zhang Z, Wang J, Li J, Wang X, Song W. MicroRNA-150 promotes cell proliferation, migration, and invasion of cervical cancer through targeting PDCD4. Biomed Pharmacother. 2018; 97:511–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CD98 activation increases surface expression and clusteringof beta 1 integrins in MCF-7 cells through FAK/Src- and cytoskeleton-independent mechanisms
- ATP-induced focal adhesion kinase activity is negatively modulated by phospholipase D2 in PC12 cells
- SUV39H1 is a New Client Protein of Hsp90 Degradated by Chaetocin as a Novel C-Terminal Inhibitor of Hsp90
- Role of Integrin, FAK (Focal Adhesion Kinase) and ERK (Extracellular Signal Regulated Kinase) on the Suppressed Cell Proliferation of Endometrial Cancer Cells by GnRH (Gonadotropin-Releasing Hormone)
- Mechanisms of Acquired Resistance to Epidermal Growth Factor Receptor Inhibitors and Overcoming Strategies in Lung Cancer