Anat Cell Biol.
2022 Jun;55(2):229-238. 10.5115/acb.21.233.
The in vitro analysis of migration and polarity of blastema cells in the extracellular matrix derived from bovine mesenteric in the presence of fibronectin
- Affiliations
-
- 1Department of Biology, Kavian Institute of Higher Education, Mashhad, Iran
- 2Department of Biology, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad, Iran
- 3Division of Biotechnology, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
- KMID: 2531209
- DOI: http://doi.org/10.5115/acb.21.233
Abstract
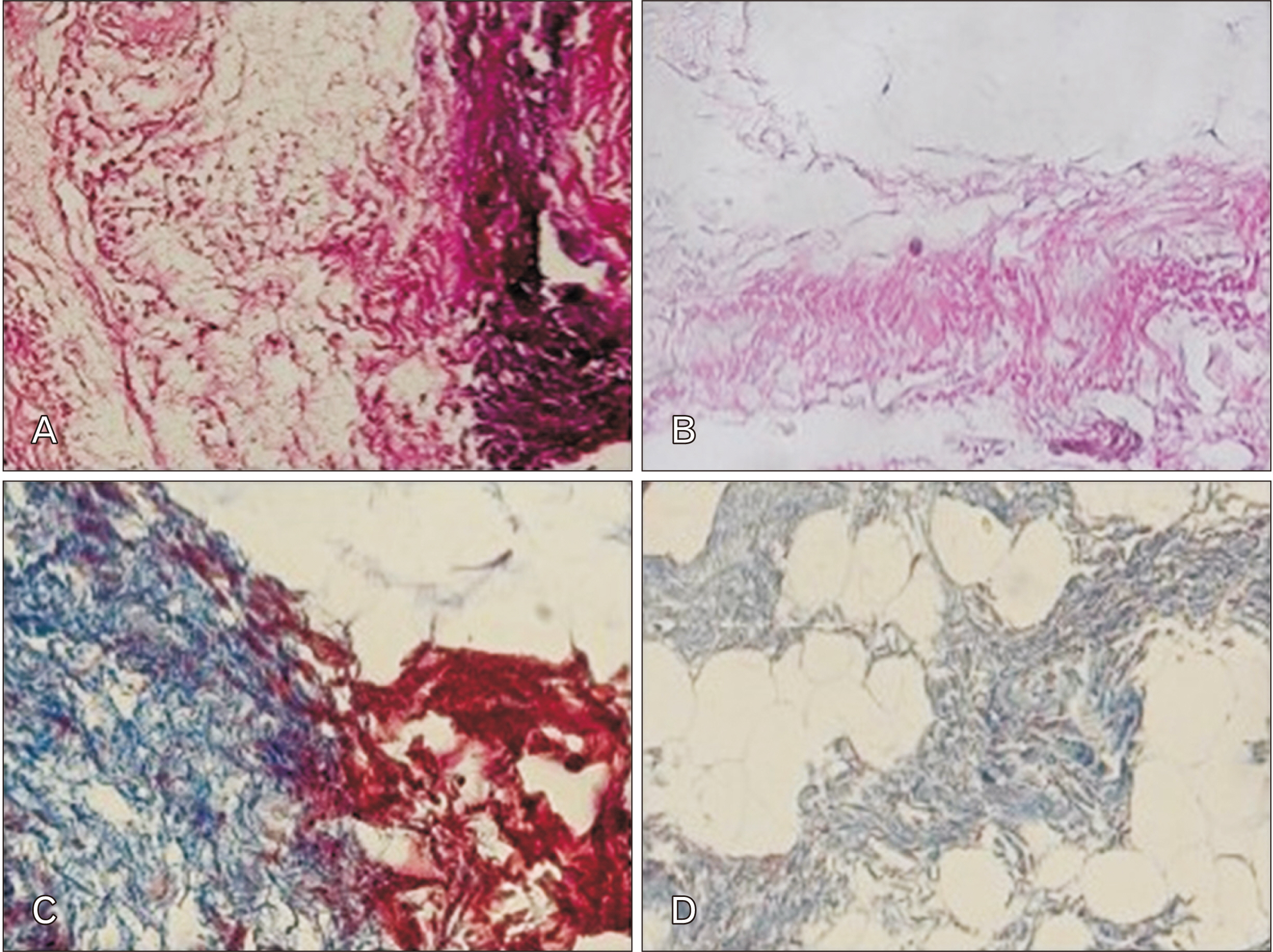
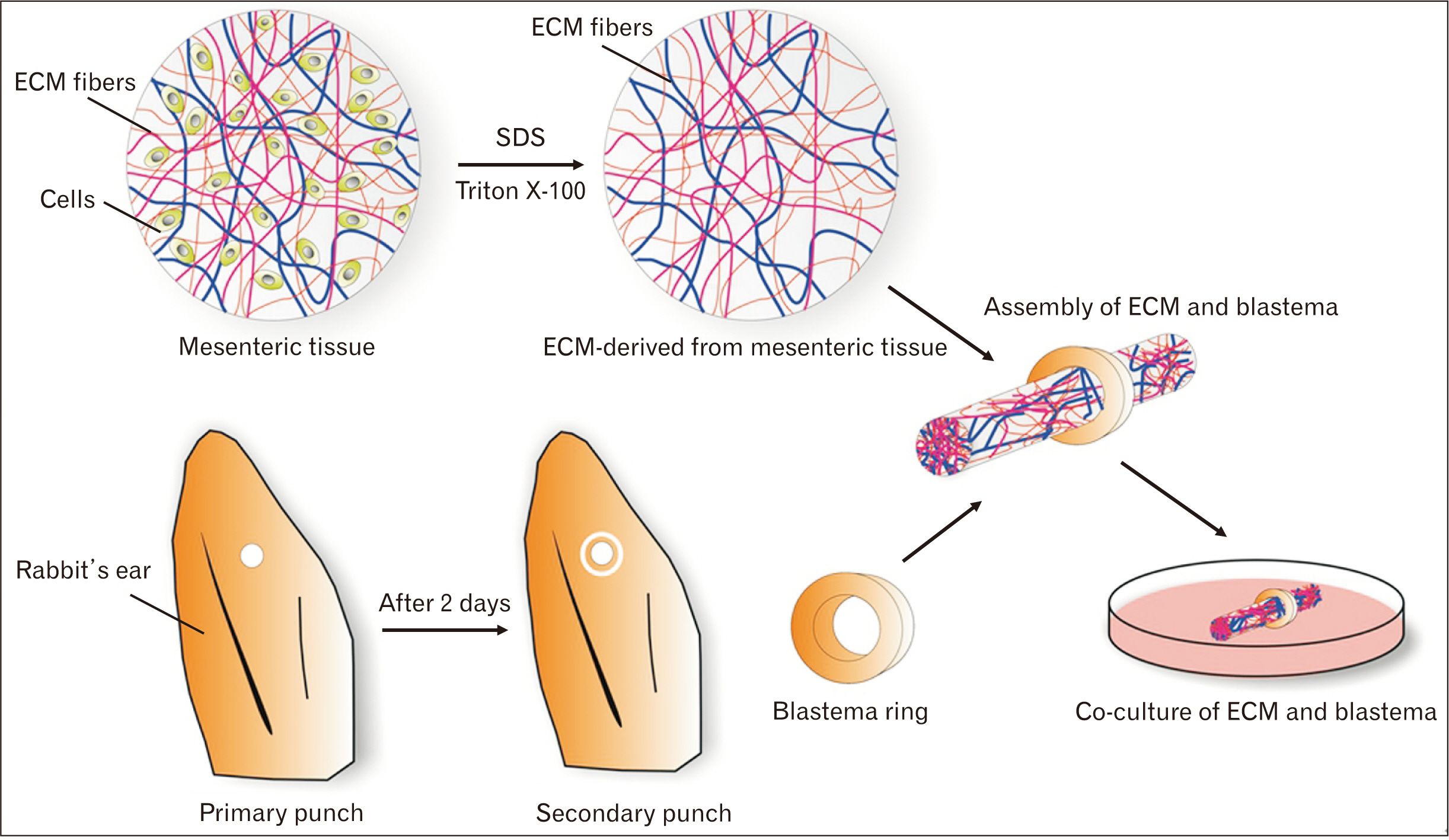
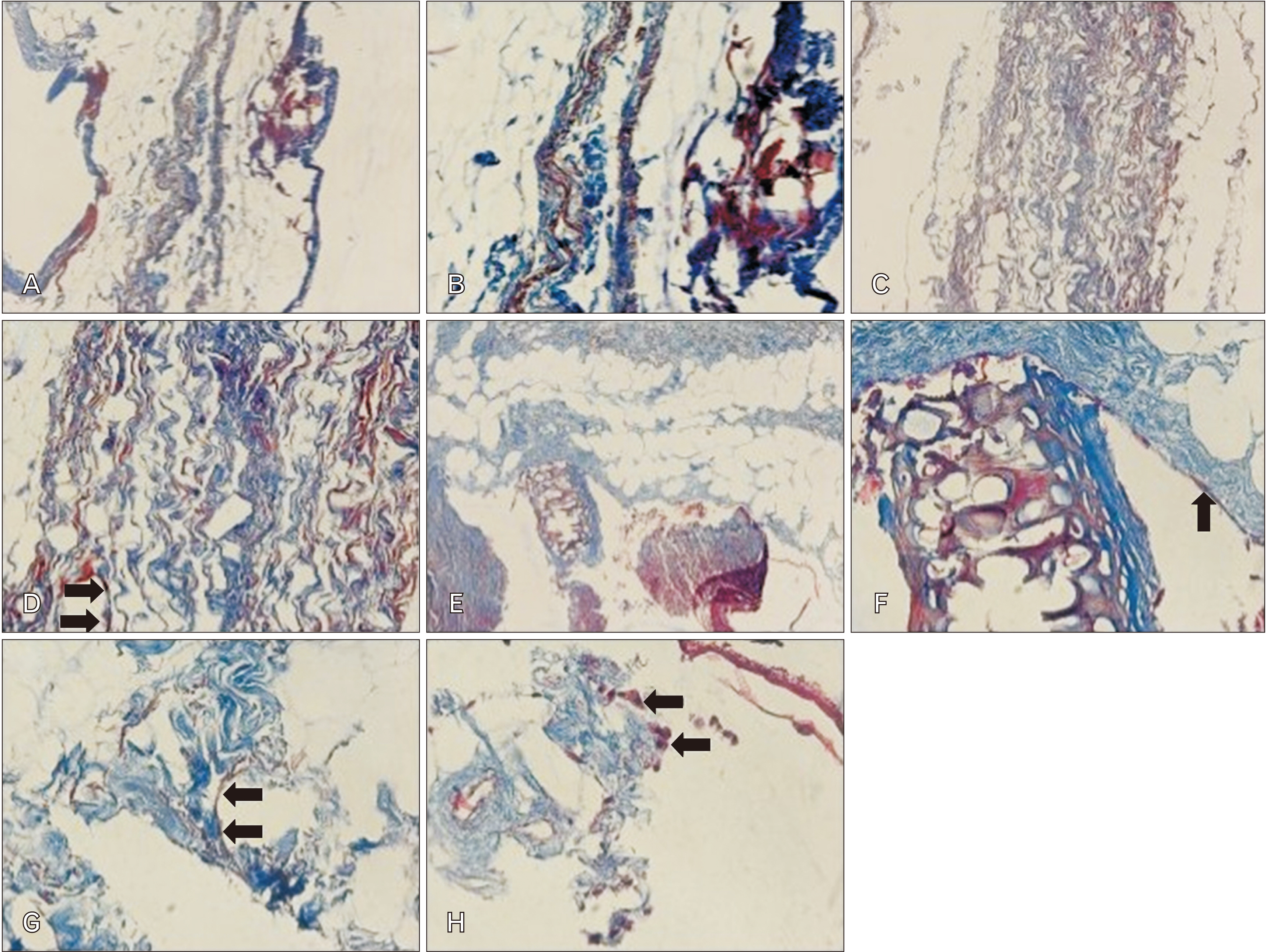
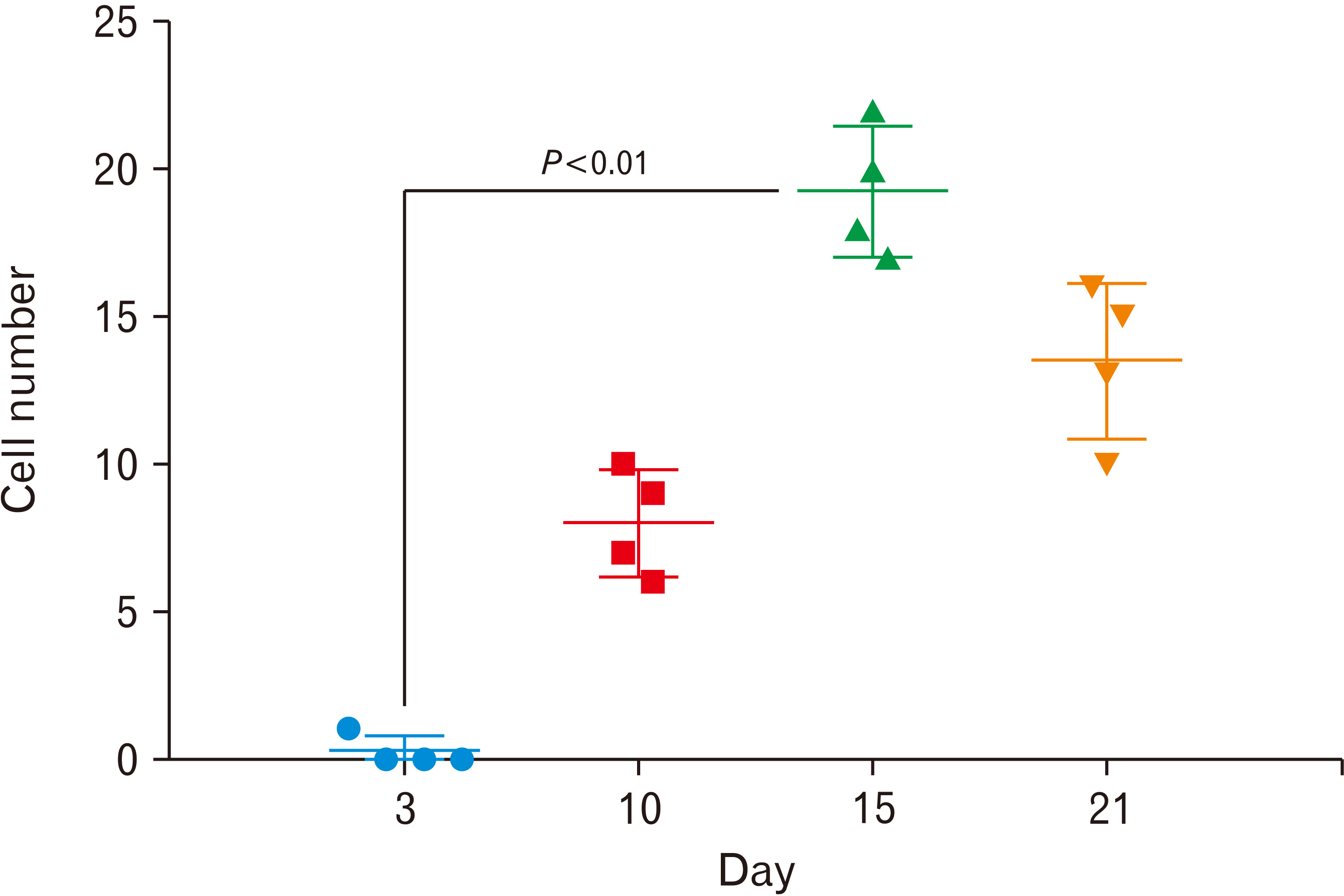
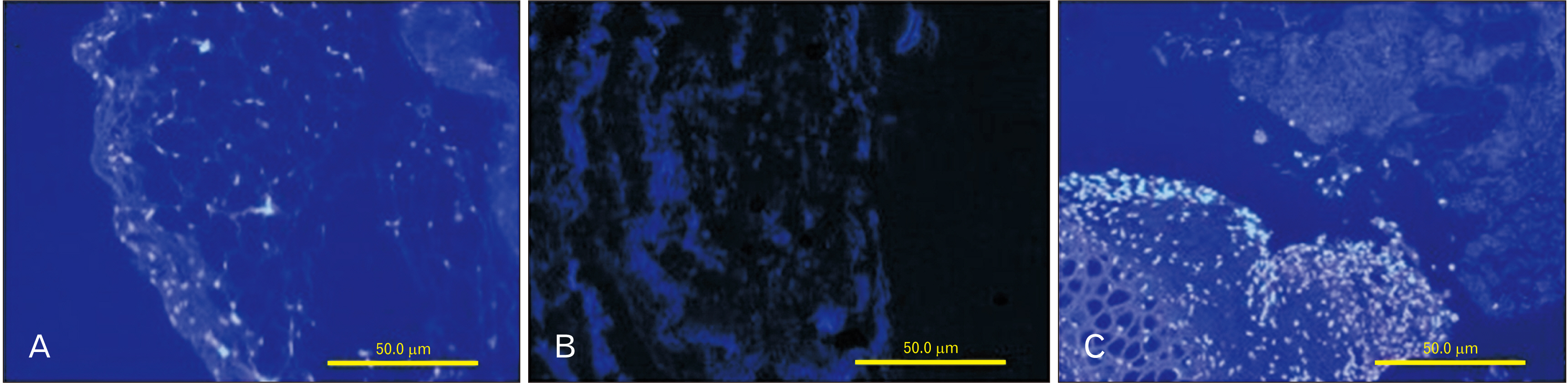
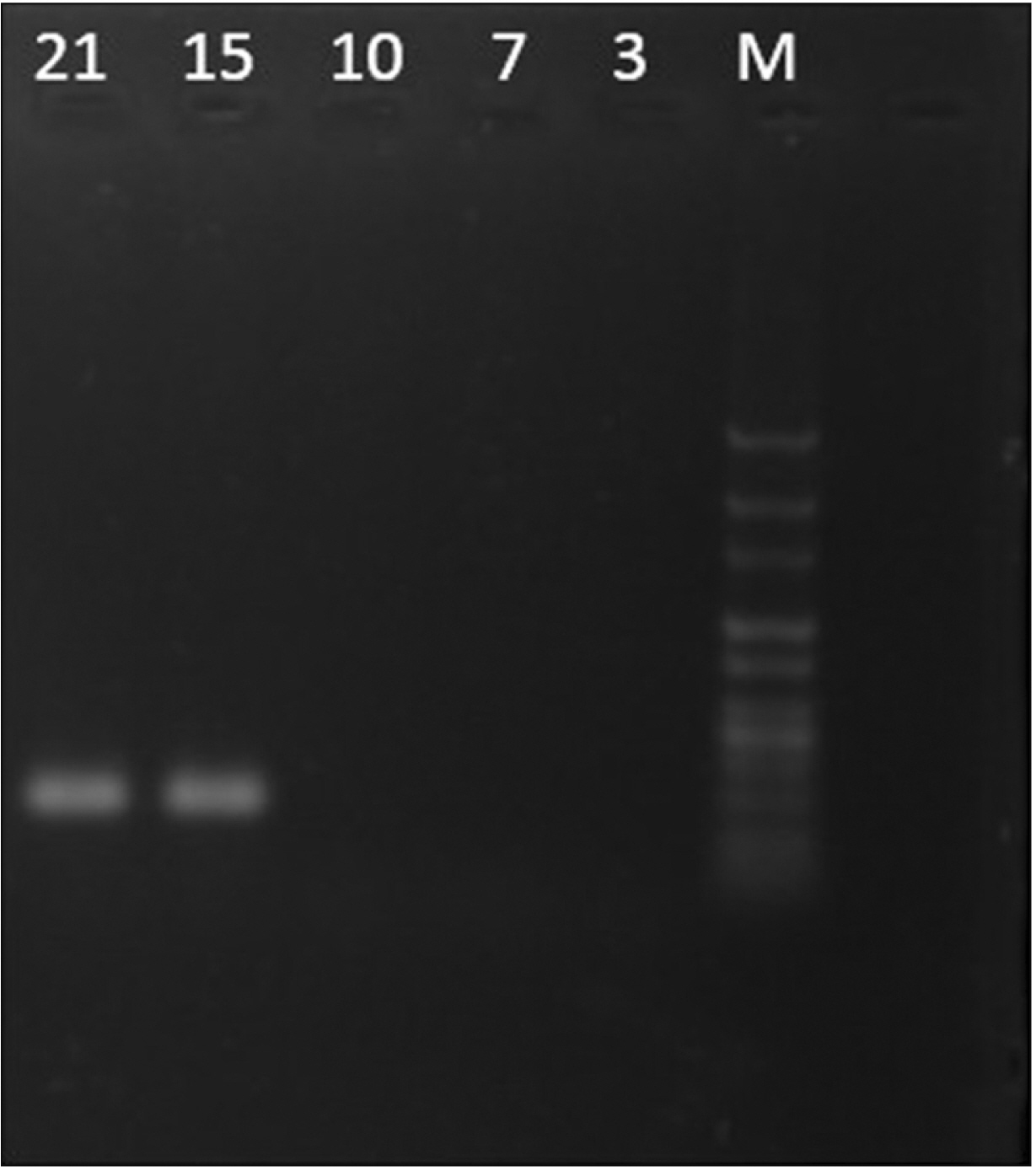
- Cell migration is an essential process in embryonic development, wound healing, and pathological conditions. Our knowledge of cell migration is often based on the two dimentional evaluation of cell movement, which usually differs from what occurred in vivo. In this study, we investigated cellular migration from blastema tissue toward bovine decellularized mesentery tissue. In this regard, fibronectin (FN) was assessed to confirm cell migration. Therefore, we established a cell migration model using blastema cells migration toward the extracellular matrix derived from bovine mesenteric tissue. A physiochemical decellularization method was utilized based on freeze-thaw cycles and agitation in sodium dodecyl sulfate and Triton X-100 to remove cells from the extracellular matrix (ECM) of bovine mesenteric tissue. These types of matrices were assembled by the rings of blastema tissues originated from the of New Zealand rabbits pinna and cultured in a medium containing FN in different days in vitro, and then they are histologically evaluated, and the expression of the Tenascin C gene is analyzed. By means of tissue staining and after confirmation of the cell removal from mesenteric tissue, polarity, and migration of blastema cells was observed in the interaction site with this matrix. Also, the expression of the Tenascin C gene was assessed on days 15 and 21 following the cell culture process. The results showed that the three dimentional model of cellular migration of blastema cells along with the ECM could be a suitable model for investigating cell behaviors, such as polarity and cell migration in vitro.
Figure
Reference
-
References
1. Charras G, Sahai E. 2014; Physical influences of the extracellular environment on cell migration. Nat Rev Mol Cell Biol. 15:813–24. DOI: 10.1038/nrm3897. PMID: 25355506.
Article2. Minuth WW, Denk L. 2015; When morphogenetic proteins encounter special extracellular matrix and cell-cell connections at the interface of the renal stem/progenitor cell niche. Anat Cell Biol. 48:1–9. DOI: 10.5115/acb.2015.48.1.1. PMID: 25806116. PMCID: PMC4371175.
Article3. Paul CD, Mistriotis P, Konstantopoulos K. 2017; Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer. 17:131–40. DOI: 10.1038/nrc.2016.123. PMID: 27909339. PMCID: PMC5364498.
Article4. Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. 2010; Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol. 26:315–33. DOI: 10.1146/annurev.cellbio.011209.122036. PMID: 19575647. PMCID: PMC4437624.
Article5. van Helvert S, Storm C, Friedl P. 2018; Mechanoreciprocity in cell migration. Nat Cell Biol. 20:8–20. DOI: 10.1038/s41556-017-0012-0. PMID: 29269951. PMCID: PMC5943039.
Article6. Friedl P, Alexander S. 2011; Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 147:992–1009. DOI: 10.1016/j.cell.2011.11.016. PMID: 22118458.
Article7. Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. 1991; Integrin alpha 2 beta 1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 115:1427–36. DOI: 10.1083/jcb.115.5.1427. PMID: 1955483. PMCID: PMC2289246.
Article8. Munevar S, Wang YL, Dembo M. 2001; Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Mol Biol Cell. 12:3947–54. DOI: 10.1091/mbc.12.12.3947. PMID: 11739792. PMCID: PMC60767.
Article9. de Almeida PG, Pinheiro GG, Nunes AM, Gonçalves AB, Thorsteinsdóttir S. 2016; Fibronectin assembly during early embryo development: a versatile communication system between cells and tissues. Dev Dyn. 245:520–35. DOI: 10.1002/dvdy.24391. PMID: 26845241.
Article10. Gilbert TW, Sellaro TL, Badylak SF. 2006; Decellularization of tissues and organs. Biomaterials. 27:3675–83. DOI: 10.1016/j.biomaterials.2006.02.014. PMID: 16519932.
Article11. Tsonis PA. 2000; Regeneration in vertebrates. Dev Biol. 221:273–84. DOI: 10.1006/dbio.2000.9667. PMID: 10790325.
Article12. Goss RJ, Grimes LN. 1975; Epidermal downgrowths in regenerating rabbit ear holes. J Morphol. 146:533–42. DOI: 10.1002/jmor.1051460408. PMID: 1171254.
Article13. Hashemzadeh MR, Mahdavi-Shahri N, Bahrami AR, Kheirabadi M, Naseri F, Atighi M. 2015; Use of an in vitro model in tissue engineering to study wound repair and differentiation of blastema tissue from rabbit pinna. In Vitro Cell Dev Biol Anim. 51:680–9. DOI: 10.1007/s11626-015-9868-0. PMID: 26091624.14. Underwood S, Afoke A, Brown RA, MacLeod AJ, Shamlou PA, Dunnill P. 2001; Wet extrusion of fibronectin-fibrinogen cables for application in tissue engineering. Biotechnol Bioeng. 73:295–305. DOI: 10.1002/bit.1062. PMID: 11283912.
Article15. Wicha P, Das S, Mahakkanukrauh P. 2021; Blood-brain barrier dysfunction in ischemic stroke and diabetes: the underlying link, mechanisms and future possible therapeutic targets. Anat Cell Biol. 54:165–77. DOI: 10.5115/acb.20.290. PMID: 33658432. PMCID: PMC8225477.
Article16. Chiquet-Ehrismann R, Chiquet M. 2003; Tenascins: regulation and putative functions during pathological stress. J Pathol. 200:488–99. DOI: 10.1002/path.1415. PMID: 12845616.
Article17. Giblin SP, Midwood KS. 2015; Tenascin-C: form versus function. Cell Adh Migr. 9:48–82. DOI: 10.4161/19336918.2014.987587. PMID: 25482829. PMCID: PMC4422809.
Article18. Midwood KS, Chiquet M, Tucker RP, Orend G. 2016; Tenascin-C at a glance. J Cell Sci. 129:4321–7. DOI: 10.1242/jcs.190546. PMID: 27875272.
Article19. Fouda GG, Jaeger FH, Amos JD, Ho C, Kunz EL, Anasti K, Stamper LW, Liebl BE, Barbas KH, Ohashi T, Moseley MA, Liao HX, Erickson HP, Alam SM, Permar SR. 2013; Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc Natl Acad Sci U S A. 110:18220–5. DOI: 10.1073/pnas.1307336110. PMID: 24145401. PMCID: PMC3831436.
Article20. Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. 1986; Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 47:131–9. DOI: 10.1016/0092-8674(86)90374-0. PMID: 2428505.
Article21. Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. 2014; Tenascins in stem cell niches. Matrix Biol. 37:112–23. DOI: 10.1016/j.matbio.2014.01.007. PMID: 24472737.
Article22. Lowy CM, Oskarsson T. 2015; Tenascin C in metastasis: a view from the invasive front. Cell Adh Migr. 9:112–24. DOI: 10.1080/19336918.2015.1008331. PMID: 25738825. PMCID: PMC4422797.
Article23. Howard CM, Baudino TA. 2014; Dynamic cell-cell and cell-ECM interactions in the heart. J Mol Cell Cardiol. 70:19–26. DOI: 10.1016/j.yjmcc.2013.10.006. PMID: 24140801.
Article24. Rosso F, Giordano A, Barbarisi M, Barbarisi A. 2004; From cell-ECM interactions to tissue engineering. J Cell Physiol. 199:174–80. DOI: 10.1002/jcp.10471. PMID: 15039999.
Article25. Yamada KM, Cukierman E. 2007; Modeling tissue morphogenesis and cancer in 3D. Cell. 130:601–10. DOI: 10.1016/j.cell.2007.08.006. PMID: 17719539.
Article26. Xu R, Zhou X, Wang S, Trinkle C. 2021; Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacol Ther. 218:107668. DOI: 10.1016/j.pharmthera.2020.107668. PMID: 32853629. PMCID: PMC7855432.
Article27. Tavassoli A, Shahabipour F, Mahdavi SN, Moghadam MM, Fereidoni M. 2010; In vitro experimental study of interactions between blastema tissue and three-dimensional matrix derived from bovine cancellous bone and articular cartilage. J Cell Tissue. 1:53–62.28. Schmidt CE, Baier JM. 2000; Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 21:2215–31. DOI: 10.1016/S0142-9612(00)00148-4. PMID: 11026628.
Article29. Schenke-Layland K, Vasilevski O, Opitz F, König K, Riemann I, Halbhuber KJ, Wahlers T, Stock UA. 2003; Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol. 143:201–8. DOI: 10.1016/j.jsb.2003.08.002. PMID: 14572475.
Article30. Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. 1995; Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 46:396–400. DOI: 10.1016/S0090-4295(99)80227-1. PMID: 7660517.
Article31. Chen RN, Ho HO, Tsai YT, Sheu MT. 2004; Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 25:2679–86. DOI: 10.1016/j.biomaterials.2003.09.070. PMID: 14751754.
Article32. Woods T, Gratzer PF. 2005; Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials. 26:7339–49. DOI: 10.1016/j.biomaterials.2005.05.066. PMID: 16023194.
Article33. Shahabipour F, Mahdavi-Shahri N, Matin MM, Tavassoli A, Zebarjad SM. 2013; Scaffolds derived from cancellous bovine bone support mesenchymal stem cells' maintenance and growth. In Vitro Cell Dev Biol Anim. 49:440–8. DOI: 10.1007/s11626-013-9591-7. PMID: 23708915.
Article34. Tavassoli A, Matin MM, Niaki MA, Mahdavi-Shahri N, Shahabipour F. 2015; Mesenchymal stem cells can survive on the extracellular matrix-derived decellularized bovine articular cartilage scaffold. Iran J Basic Med Sci. 18:1221–7. PMID: 26877852. PMCID: PMC4744362.35. Maurer LM, Annis DS, Mosher DF. 2012; IGD motifs, which are required for migration stimulatory activity of fibronectin type I modules, do not mediate binding in matrix assembly. PLoS One. 7:e30615. DOI: 10.1371/journal.pone.0030615. PMID: 22355321. PMCID: PMC3280255.
Article36. Arredondo R, Poggioli F, Martínez-Díaz S, Piera-Trilla M, Torres-Claramunt R, Tío L, Monllau JC. 2021; Fibronectin-coating enhances attachment and proliferation of mesenchymal stem cells on a polyurethane meniscal scaffold. Regen Ther. 18:480–6. DOI: 10.1016/j.reth.2021.11.001. PMID: 34926733. PMCID: PMC8633527.
Article37. Van Obberghen-Schilling E, Tucker RP, Saupe F, Gasser I, Cseh B, Orend G. 2011; Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. Int J Dev Biol. 55:511–25. DOI: 10.1387/ijdb.103243eo. PMID: 21769776.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular matrix of the human retinal pigment epithelial cells in vitro
- Extracellular Matrix of the Cultured Retinal Pigment Epithelial Cells
- The changes of fibronectin in the cultured porcine lens epithelial cells
- Morphological Change and Distribution of Laminin and Fibronectin in Early Chick Embryos
- The Effects of Various Extracellular Matrices on Motility of Cultured MC3T3-E1 Cell