Diabetes Metab J.
2022 Mar;46(2):337-348. 10.4093/dmj.2021.0056.
DA-1241, a Novel GPR119 Agonist, Improves Hyperglycaemia by Inhibiting Hepatic Gluconeogenesis and Enhancing Insulin Secretion in Diabetic Mice
- Affiliations
-
- 1Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- 2Graduate School of Medicine, Yonsei University, Seoul, Korea
- 3Department of Pharmacology, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Clinical Nursing Science, Yonsei University College of Nursing, Seoul, Korea
- KMID: 2527726
- DOI: http://doi.org/10.4093/dmj.2021.0056
Abstract
- Background
We investigated the antidiabetic effects of DA-1241, a novel G protein-coupled receptor (GPR) 119 agonist, in vitro and in vivo.
Methods
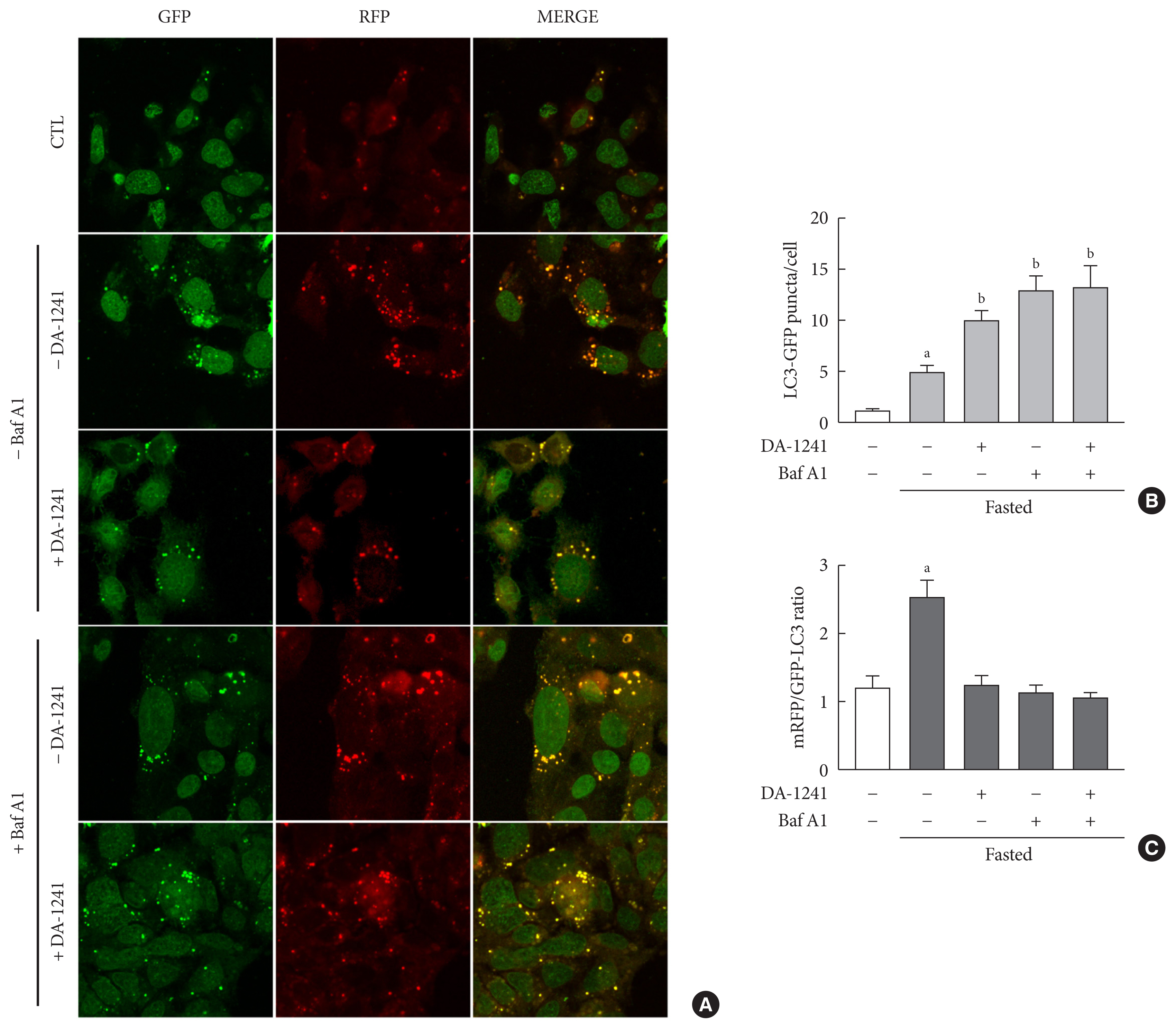
DA-1241 was administrated to high-fat diet (HFD)-fed C57BL/6J mice for 12 weeks after hyperglycaemia developed. Oral/intraperitoneal glucose tolerance test and insulin tolerance test were performed. Serum insulin and glucagon-like peptide-1 (GLP-1) levels were measured during oral glucose tolerance test. Insulinoma cell line (INS-1E) cells and mouse islets were used to find whether DA-1241 directly stimulate insulin secretion in beta cell. HepG2 cells were used to evaluate the gluconeogenesis and autophagic process. Autophagic flux was evaluated by transfecting microtubule-associated protein 1 light chain 3-fused to green fluorescent protein and monomeric red fluorescent (mRFP-GFP-LC3) expression vector to HepG2 cells.
Results
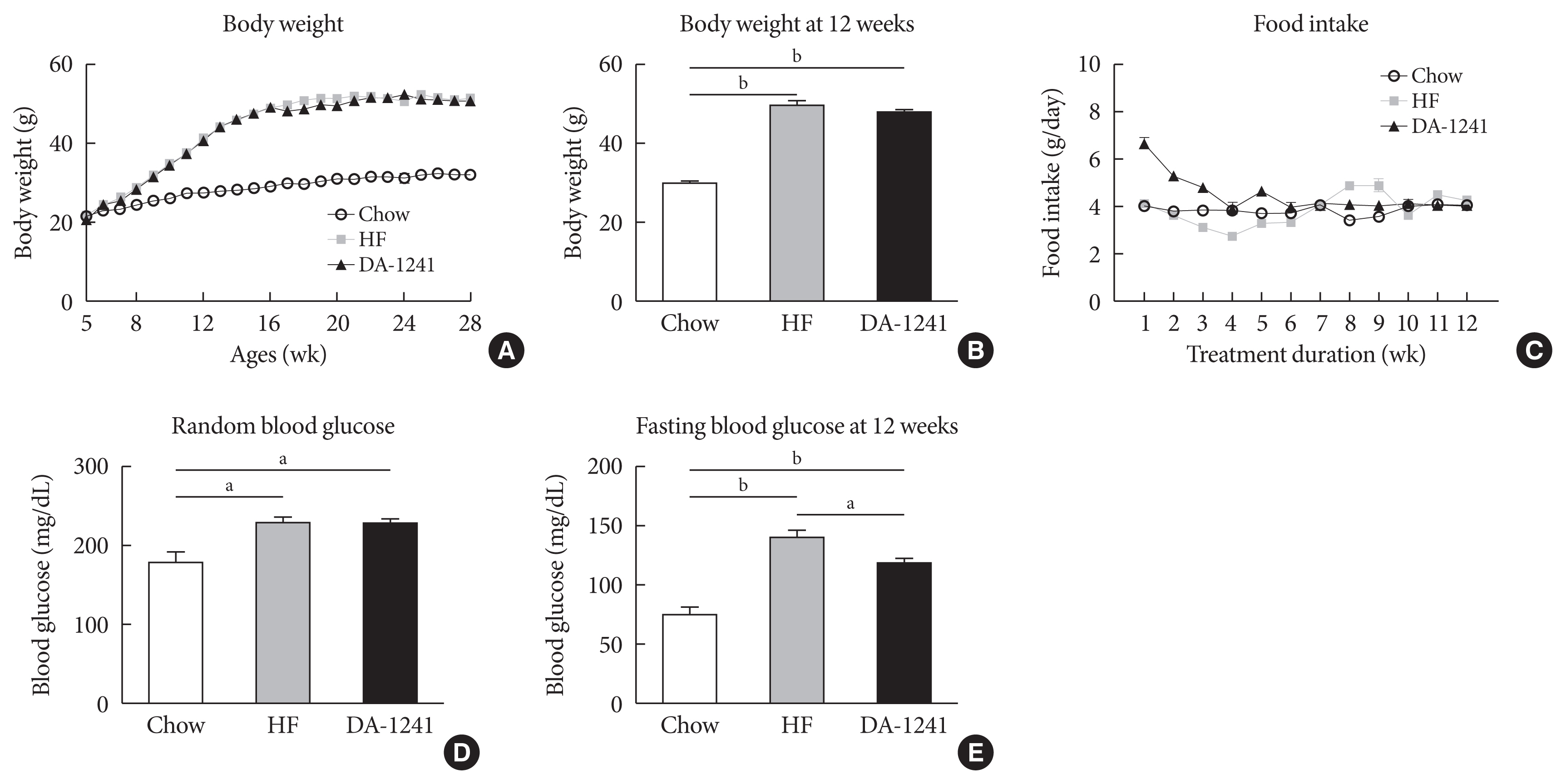
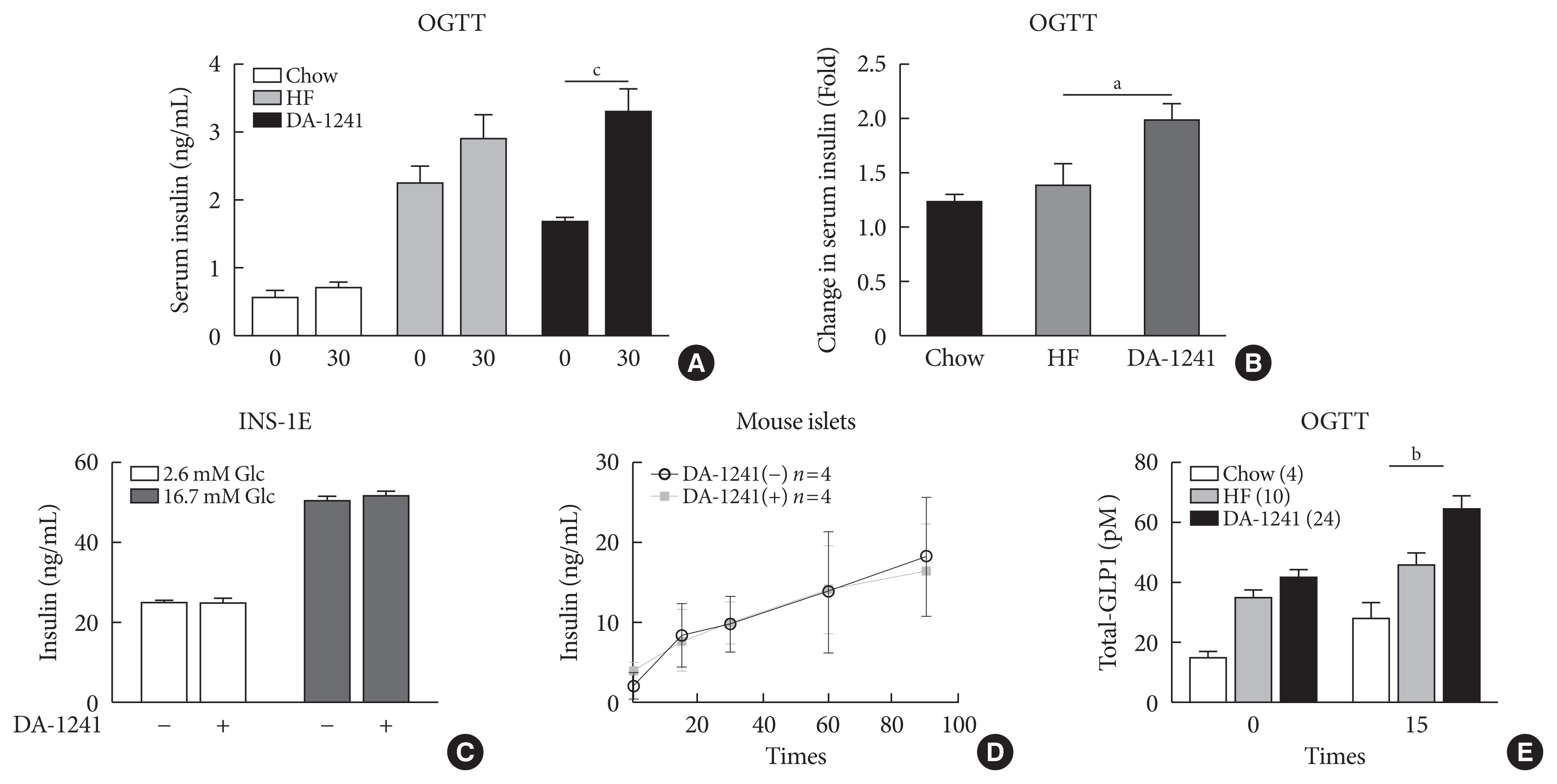
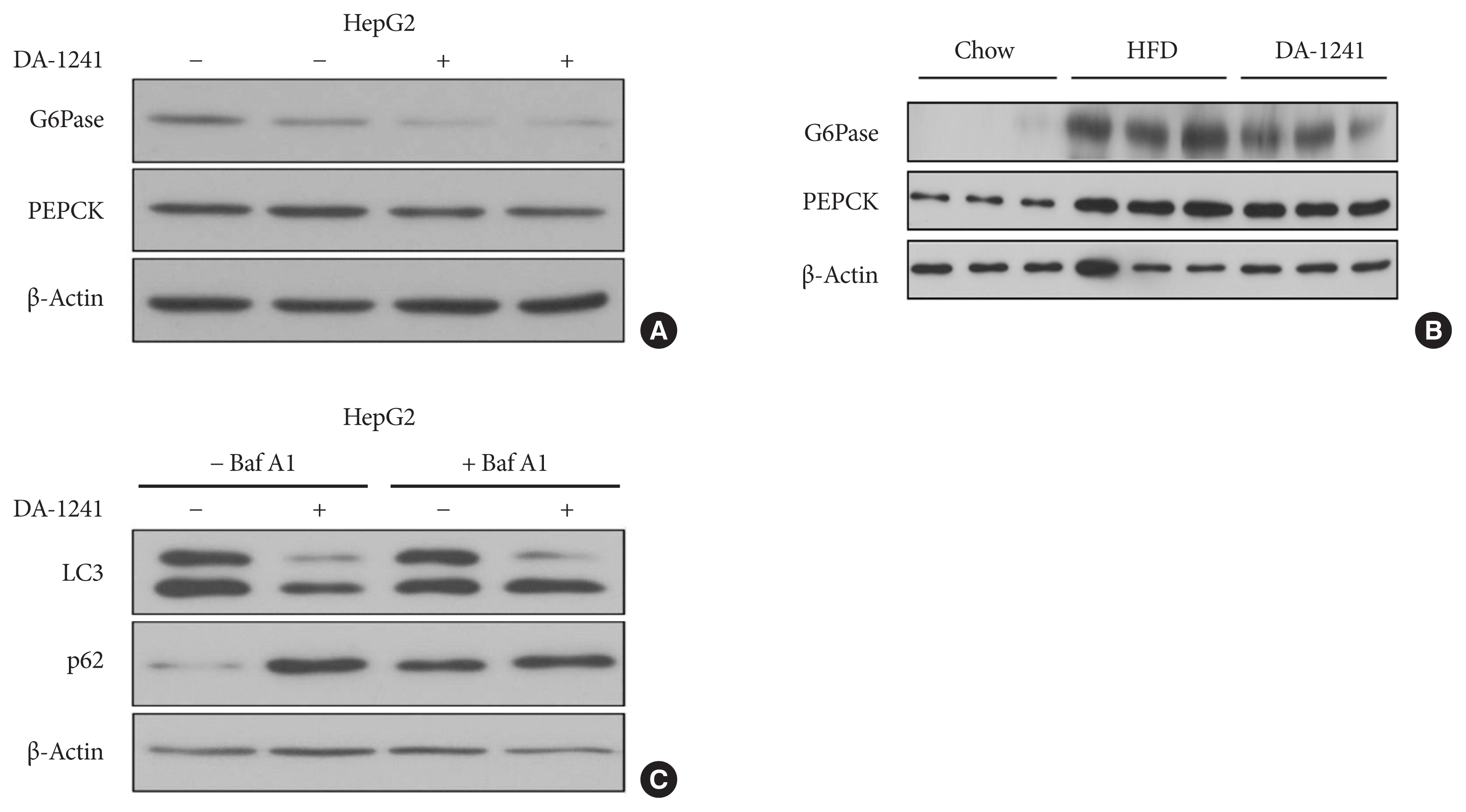
Although DA-1241 treatment did not affect body weight gain and amount of food intake, fasting blood glucose level decreased along with increase in GLP-1 level. DA-1241 improved only oral glucose tolerance test and showed no effect in intraperitoneal glucose tolerance test. No significant effect was observed in insulin tolerance test. DA-1241 did not increase insulin secretion in INS-1E cell and mouse islets. DA-1241 reduced triglyceride content in the liver thereby improved fatty liver. Additionally, DA-1241 reduced gluconeogenic enzyme expression in HepG2 cells and mouse liver. DA-1241 reduced autophagic flow in HepG2 cells.
Conclusion
These findings suggested that DA-1241 augmented glucose-dependent insulin release via stimulation of GLP-1 secretion, and reduced hepatic gluconeogenesis, which might be associated with autophagic blockage, leading to improved glycaemic control.
Keyword
Figure
Reference
-
1. Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005; 365:1333–46.
Article2. Shah U, Kowalski TJ. GPR119 agonists for the potential treatment of type 2 diabetes and related metabolic disorders. Vitam Horm. 2010; 84:415–48.
Article3. Overton HA, Fyfe MC, Reynet C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol. 2008; 153(Suppl 1):S76–81.
Article4. Jones RM, Leonard JN, Buzard DJ, Lehmann J. GPR119 agonists for the treatment of type 2 diabetes. Expert Opin Ther Pat. 2009; 19:1339–59.
Article5. Yang JW, Kim HS, Choi YW, Kim YM, Kang KW. Therapeutic application of GPR119 ligands in metabolic disorders. Diabetes Obes Metab. 2018; 20:257–69.
Article6. Soga T, Ohishi T, Matsui T, Saito T, Matsumoto M, Takasaki J, et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun. 2005; 326:744–51.
Article7. Chu ZL, Jones RM, He H, Carroll C, Gutierrez V, Lucman A, et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology. 2007; 148:2601–9.8. Chu ZL, Carroll C, Alfonso J, Gutierrez V, He H, Lucman A, et al. A role for intestinal endocrine cell-expressed g protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology. 2008; 149:2038–47.
Article9. Kang SU. GPR119 agonists: a promising approach for T2DM treatment?: a SWOT analysis of GPR119. Drug Discov Today. 2013; 18:1309–15.
Article10. Kim M, Kim T, Choeng Y, Chae Y, Jung I, Lee K, et al. Long-term treatment of DA-1241, a novel GPR119 agonist, improved glucose control via preserved beta cell mass in a progressive diabetic mice model. Diabetes. 2015; 64(Suppl 1):280. LB.11. Kim MK, Kim TH, Lee S, Jung IH, Chea YN, Yang JS. Effects of DA-1241, a novel GPR-119 agonist, on lipid control in disease models mediated by regulating an AMPK/SREBP1c signaling path. Diabetes. 2017; 66(Suppl 1):161. LB.12. Kim YJ, Kim RH, Park HK, Cho YI, Lee MY, Lee JY, et al. 139-PE: DA-1241, a novel GPR119 agonist, improves glycemia via inhibition of hepatic gluconeogenesis and enhancing glucose stimulated insulin action via stimulating GLP-1 secretion in intestine. In : Proceedings of International Congress of Diabetes and Metabolism 2018; 2018 Oct 11–13; Seoul, KR.13. Rui L. Energy metabolism in the liver. Compr Physiol. 2014; 4:177–97.
Article14. Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age? World J Gastroenterol. 2014; 20:9072–89.15. Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012; 56:952–64.
Article16. Ezaki J, Matsumoto N, Takeda-Ezaki M, Komatsu M, Takahashi K, Hiraoka Y, et al. Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy. 2011; 7:727–36.
Article17. Wang HJ, Park JY, Kwon O, Choe EY, Kim CH, Hur KY, et al. Chronic HMGCR/HMG-CoA reductase inhibitor treatment contributes to dysglycemia by upregulating hepatic gluconeogenesis through autophagy induction. Autophagy. 2015; 11:2089–101.
Article18. Jeon JY, Lee H, Park J, Lee M, Park SW, Kim JS, et al. The regulation of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase by autophagy in low-glycolytic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2015; 463:440–6.
Article19. Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus: a 13C nuclear magnetic resonance study. J Clin Invest. 1992; 90:1323–7.
Article20. Li DS, Yuan YH, Tu HJ, Liang QL, Dai LJ. A protocol for islet isolation from mouse pancreas. Nat Protoc. 2009; 4:1649–52.
Article21. Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015; 19:160–4.
Article22. Patarrao RS, Lautt WW, Macedo MP. Assessment of methods and indexes of insulin sensitivity. Rev Port Endocrinol Diabetes Metab. 2014; 9:65–73.
Article23. Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007; 3:542–5.
Article24. Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci. 2017; 18:1865.
Article25. Odori S, Hosoda K, Tomita T, Fujikura J, Kusakabe T, Kawaguchi Y, et al. GPR119 expression in normal human tissues and islet cell tumors: evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism. 2013; 62:70–8.
Article26. Moran BM, Abdel-Wahab YH, Flatt PR, McKillop AM. Activation of GPR119 by fatty acid agonists augments insulin release from clonal β-cells and isolated pancreatic islets and improves glucose tolerance in mice. Biol Chem. 2014; 395:453–64.
Article27. Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987; 84:3434–8.
Article28. Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009; 58:1058–66.
Article29. Kim SR, Kim DH, Park SH, Kim YS, Kim CH, Ha TY, et al. In vivo efficacy of HD0471953: a novel GPR119 agonist for the treatment of type 2 diabetes mellitus. J Diabetes Res. 2013; 2013:269569.
Article30. Skelin M, Rupnik M, Cencic A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX. 2010; 27:105–13.
Article31. Han T, Lee BM, Park YH, Lee DH, Choi HH, Lee T, et al. YH18968, a novel 1,2,4-triazolone G-protein coupled receptor 119 agonist for the treatment of type 2 diabetes mellitus. Biomol Ther (Seoul). 2018; 26:201–9.
Article32. Stone VM, Dhayal S, Smith DM, Lenaghan C, Brocklehurst KJ, Morgan NG. The cytoprotective effects of oleoylethanolamide in insulin-secreting cells do not require activation of GPR119. Br J Pharmacol. 2012; 165:2758–70.
Article33. Flock G, Holland D, Seino Y, Drucker DJ. GPR119 regulates murine glucose homeostasis through incretin receptor-dependent and independent mechanisms. Endocrinology. 2011; 152:374–83.
Article34. Panaro BL, Flock GB, Campbell JE, Beaudry JL, Cao X, Drucker DJ. β-Cell inactivation of Gpr119 unmasks incretin dependence of GPR119-mediated glucoregulation. Diabetes. 2017; 66:1626–35.35. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006; 3:153–65.
Article36. Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996; 379:69–72.
Article37. Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006; 3:167–75.
Article38. Ning Y, O’Neill K, Lan H, Pang L, Shan LX, Hawes BE, et al. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol. 2008; 155:1056–65.
Article39. Bahirat UA, Shenoy RR, Goel RN, Nemmani KV. APD668, a G protein-coupled receptor 119 agonist improves fat tolerance and attenuates fatty liver in high-trans fat diet induced steatohepatitis model in C57BL/6 mice. Eur J Pharmacol. 2017; 801:35–45.
Article40. Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011; 54:1214–23.
Article41. Yang JW, Kim HS, Im JH, Kim JW, Jun DW, Lim SC, et al. GPR119: a promising target for nonalcoholic fatty liver disease. FASEB J. 2016; 30:324–35.
Article42. Eberle D, Hegarty B, Bossard P, Ferre P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004; 86:839–48.
Article43. Yamamoto T, Shimano H, Inoue N, Nakagawa Y, Matsuzaka T, Takahashi A, et al. Protein kinase A suppresses sterol regulatory element-binding protein-1C expression via phosphorylation of liver X receptor in the liver. J Biol Chem. 2007; 282:11687–95.
Article44. Arguello G, Balboa E, Arrese M, Zanlungo S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim Biophys Acta. 2015; 1852:1765–78.
Article45. Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013; 19:83–92.
Article46. Shibata M, Yoshimura K, Furuya N, Koike M, Ueno T, Komatsu M, et al. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem Biophys Res Commun. 2009; 382:419–23.
Article47. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, et al. Autophagy regulates lipid metabolism. Nature. 2009; 458:1131–5.
Article48. Kwanten WJ, Martinet W, Michielsen PP, Francque SM. Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: a controversial issue. World J Gastroenterol. 2014; 20:7325–38.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HD047703, a New Promising Anti-Diabetic Drug Candidate: In Vivo Preclinical Studies
- Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- YH18968, a Novel 1,2,4-Triazolone G-Protein Coupled Receptor 119 Agonist for the Treatment of Type 2 Diabetes Mellitus
- Hepatic Expression of the Serine Palmitoyltransferase Subunit Sptlc2 Reduces Lipid Droplets in the Liver by Activating VLDL Secretion
- Aerobic Exercise and Metformin: A Dual Approach to Enhancing Glycemic Maintenance in Type 2 Diabetes Mellitus