Clin Endosc.
2022 Mar;55(2):256-262. 10.5946/ce.2021.061.
Value of Fecal Calprotectin Measurement During the Initial Period of Therapeutic Anti-Tubercular Trial
- Affiliations
-
- 1Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 2Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea
- 3Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 4Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2527570
- DOI: http://doi.org/10.5946/ce.2021.061
Abstract
- Background/Aims
The diagnosis of intestinal tuberculosis (Itbc) is often challenging. Therapeutic anti-tubercular trial (TATT) is sometimes used for the diagnosis of Itbc. We aimed to evaluate the changing pattern of fecal calprotectin (FC) levels during TATT in patients with Itbc.
Methods
A retrospective review was performed on the data of 39 patients who underwent TATT between September 2015 and November 2018 in five university hospitals in Daegu, South Korea. The analysis was performed for 33 patients with serial FC measurement reports.
Results
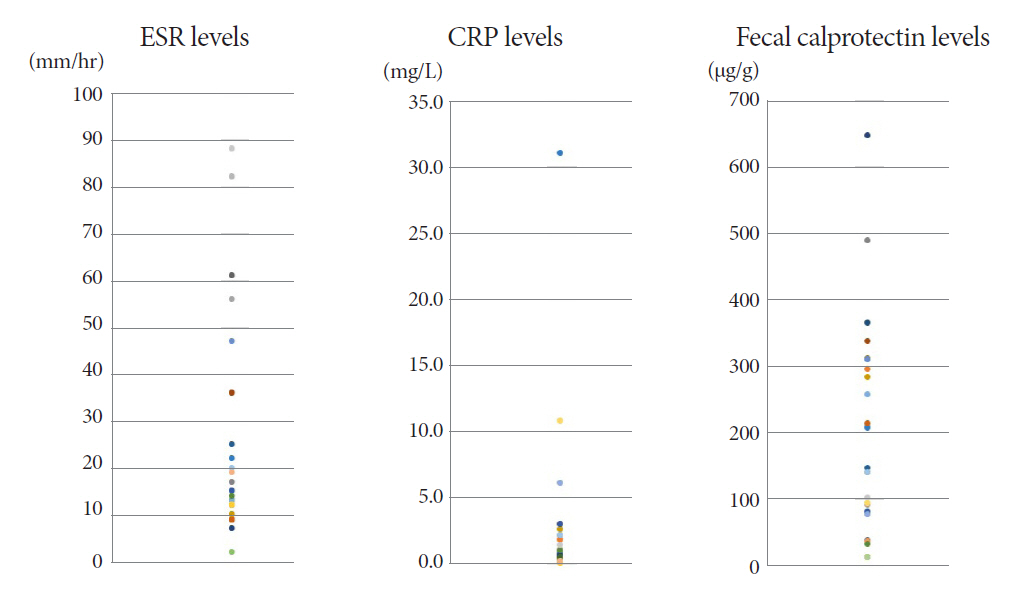
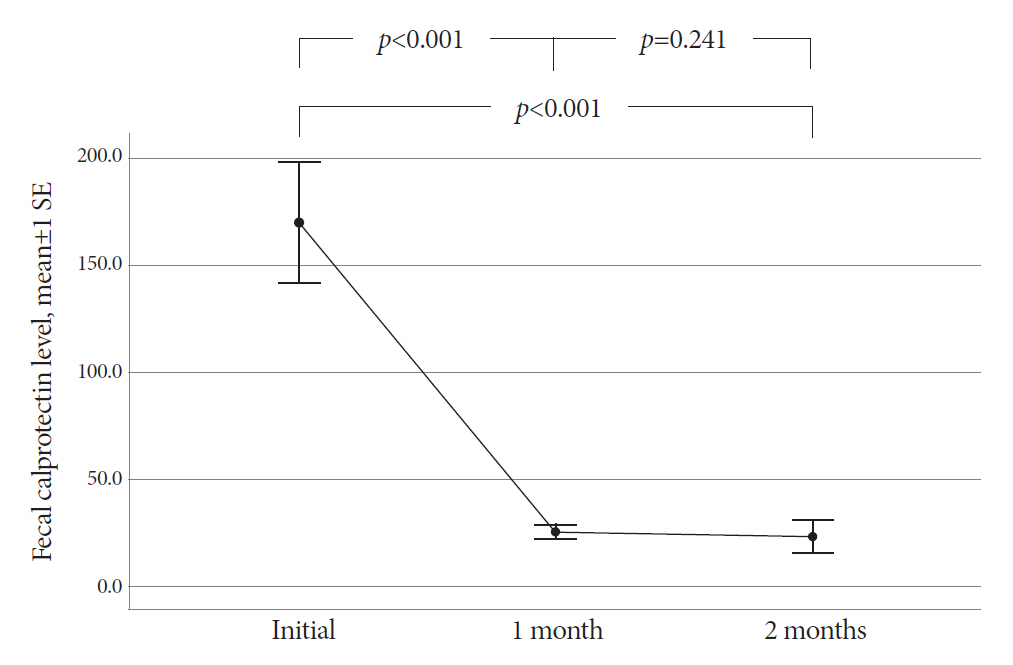
The mean age of the participants was 48.8 years. The final diagnosis of Itbc was confirmed in 30 patients based on complete mucosal healing on follow-up colonoscopy performed after 2 months of TATT. Before starting TATT, the mean FC level of the Itbc patients was 170.2 μg/g (range, 11.5-646.5). It dropped to 25.4 μg/g (range, 11.5-75.3) and then 23.3 μg/g (range, 11.5-172.2) after one and two months of TATT, respectively. The difference in mean FC before and one month after TATT was statistically significant (p<0.001), and FC levels decreased to below 100 μg/g in all patients after one month of TATT.
Conclusions
All Itbc patients showed FC decline after only 1 month of TATT, and this finding correlated with complete mucosal healing in the follow-up colonoscopy after 2 months of TATT.
Figure
Reference
-
1. Jin XJ, Kim JM, Kim HK, et al. Histopathology and TB-PCR kit analysis in differentiating the diagnosis of intestinal tuberculosis and Crohn’s disease. World J Gastroenterol. 2010; 16:2496–2503.2. Ooi CJ, Makharia GK, Hilmi I, et al. Asia Pacific Consensus Statements on Crohn’s disease. Part 1: definition, diagnosis, and epidemiology: (asia pacific Crohn’s disease consensus--Part 1). J Gastroenterol Hepatol. 2016; 31:45–55.3. Pathirana WGW, Chubb SP, Gillett MJ, Vasikaran SD. Faecal calprotectin. Clin Biochem Rev. 2018; 39:77–90.4. Dutta AK, Sahu MK, Gangadharan SK, Chacko A. Distinguishing Crohn’s disease from intestinal tuberculosis--a prospective study. Trop Gastroenterol. 2011; 32:204–209.5. Li Y, Zhang L, Liu X, et al. The role of in vitro interferonγ-release assay in differentiating intestinal tuberculosis from Crohn’s disease in China. J Crohns Colitis. 2012; 6:317–323.6. Lei Y, Yi FM, Zhao J, et al. Utility of in vitro interferon-γ release assay in differential diagnosis between intestinal tuberculosis and Crohn’s disease. J Dig Dis. 2013; 14:68–75.7. Ramadass B, Chittaranjan S, Subramanian V, Ramakrishna BS. Fecal polymerase chain reaction for Mycobacterium tuberculosis IS6110 to distinguish Crohn’s disease from intestinal tuberculosis. Indian J Gastroenterol. 2010; 29:152–156.8. Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn’s disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008; 14:741–746.9. Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn’s disease and intestinal tuberculosis. Am J Gastroenterol. 2010; 105:642–651.10. Fei BY, Lv HX, Zheng WH. Fluorescent quantitative PCR of Mycobacterium tuberculosis for differentiating intestinal tuberculosis from Crohn’s disease. Braz J Med Biol Res. 2014; 47:166–170.11. Kim YS, Kim YH, Lee KM, Kim JS, Park YS, IBD Study Group of the Korean Association of the Study of Intestinal Diseases. Diagnostic guideline of intestinal tuberculosis. Korean J Gastroenterol. 2009; 53:177–186.12. Chen W, Fan JH, Luo W, Peng P, Su SB. Effectiveness of interferon-gamma release assays for differentiating intestinal tuberculosis from Crohn’s disease: a meta-analysis. World J Gastroenterol. 2013; 19:8133–8140.13. Yamasue M, Komiya K, Usagawa Y, et al. Factors associated with false negative interferon-γ release assay results in patients with tuberculosis: a systematic review with meta-analysis. Sci Rep. 2020; 10:1607.14. Seo H, Lee S, So H, et al. Temporal trends in the misdiagnosis rates between Crohn’s disease and intestinal tuberculosis. World J Gastroenterol. 2017; 23:6306–6314.15. Lee KM. Fecal biomarkers in inflammatory bowel disease. Intest Res. 2013; 11:73–78.16. Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2006; 12:524–534.17. Lee YW, Lee KM, Lee JM, et al. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J Intern Med. 2019; 34:72–80.18. Mumolo MG, Bertani L, Ceccarelli L, et al. From bench to bedside: fecal calprotectin in inflammatory bowel diseases clinical setting. World J Gastroenterol. 2018; 24:3681–3694.19. Sharma V, Verma S, Kumar-M P, et al. Serial measurements of faecal calprotectin may discriminate intestinal tuberculosis and Crohn’s disease in patients started on antitubercular therapy. Eur J Gastroenterol Hepatol. 2021; 33:334–338.20. Desai D, Faubion WA, Sandborn WJ. Review article: biological activity markers in inflammatory bowel disease. Aliment Pharmacol Ther. 2007; 25:247–255.21. Lasson A, Stotzer PO, Öhman L, Isaksson S, Sapnara M, Strid H. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. 2015; 9:26–32.22. Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009; 44:879–888.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fecal Calprotectin as a Surrogate Marker for Mucosal Healing After Initiating the Therapeutic Anti-Tubercular Trial
- Accuracy of three different fecal calprotectin tests in the diagnosis of inflammatory bowel disease
- Clinical Utility of Fecal Neutrophil Gelatinase-Associated Lipocalin and Calprotectin as Biomarkers of Clostridioides (Clostridium) difficile Infection
- Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis
- Fecal calprotectin concentration in neonatal necrotizing enterocolitis