J Korean Neurosurg Soc.
2022 Mar;65(2):180-185. 10.3340/jkns.2021.0077.
Neurotoxicity of Paclitaxel and Rapamycin in a Rat Model with Transient Blood-Brain Barrier Opening
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea
- 2Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea
- 3Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2527174
- DOI: http://doi.org/10.3340/jkns.2021.0077
Abstract
Objective
: Drug-eluting stents and balloons are occasionally used to reduce restenosis in medically intractable intracranial atherosclerotic stenosis. The authors aimed to determine whether such drugs can cause neurotoxicity due to local effects in a rat model.
Methods
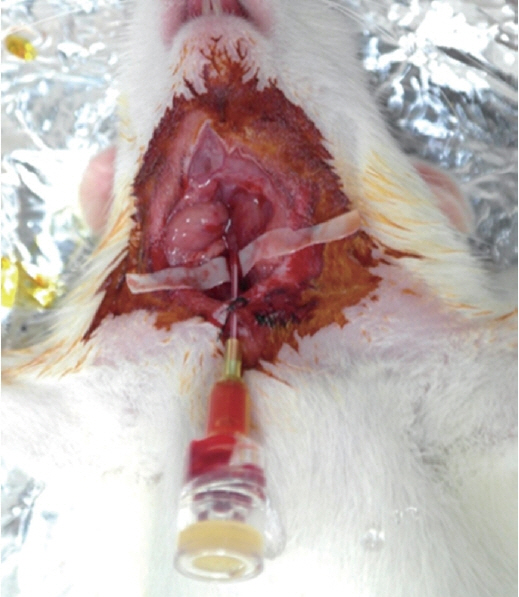
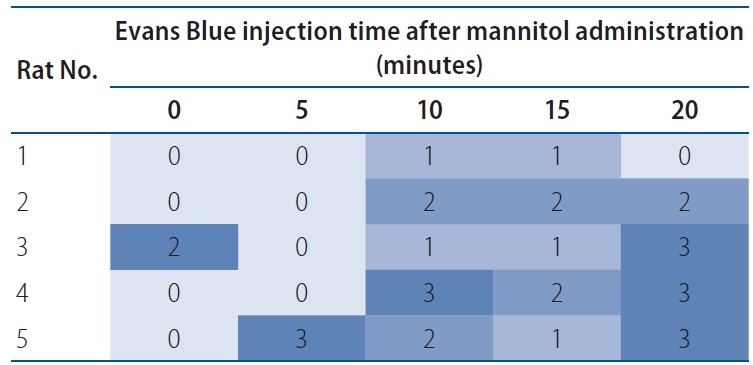
: Intra-arterial catheters were placed in the right common carotid artery of rats. Mannitol was injected to transiently open the brain-blood barrier (BBB), followed by high-dose drug (paclitaxel and rapamycin) injection. The optimal time interval of transient BBB opening for maximal drug penetration was determined to be 10 minutes. Paclitaxel and rapamycin were intraarterially administered in various doses. All the rats were neurologically evaluated, and their brain tissues were histologically examined.
Results
: Neither neurological deficits nor histological abnormalities were observed in all the rats.
Conclusion
: Paclitaxel and rapamycin did not cause neurotoxicity in a rat model with transient BBB opening.
Figure
Reference
-
References
1. Abou-Chebl A, Bashir Q, Yadav JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. 36:e165–e168. 2005.2. Barnard ZR, Alexander MJ. Update in the treatment of intracranial atherosclerotic disease. Stroke Vasc Neurol. 5:59–64. 2019.
Article3. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 17:472–476. 1986.
Article4. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 365:993–1003. 2011.5. Gheith O, Cerna M, Halim MA, Nampoory N, Al-Otaibi T, Nair P, et al. Sirolimus-induced combined posterior reversible encephalopathy syndrome and lymphocytic pneumonitis in a renal transplant recipient: case report and review of the literature. Exp Clin Transplant. 15(Suppl 1):170–174. 2017.6. Gupta R, Al-Ali F, Thomas AJ, Horowitz MB, Barrow T, Vora NA, et al. Safety, feasibility, and short-term follow-up of drug-eluting stent placement in the intracranial and extracranial circulation. Stroke. 37:2562–2566. 2006.
Article7. Kim J, Ban SP, Kim YD, Kwon OK. Long-term outcomes of drug-eluting stent implantation in patients with symptomatic extra- and intracranial atherosclerotic stenoses. J Cerebrovasc Endovasc Neurosurg. 22:216–224. 2020.
Article8. Kim SJ, Kim YJ, Ko JH. Long term outcome of in-stent stenosis after stent assisted coil embolization for cerebral aneurysm. J Korean Neurosurg Soc. 62:536–544. 2019.
Article9. Levy EI, Hanel RA, Howington JU, Nemes B, Boulos AS, Tio FO, et al. Sirolimus-eluting stents in the canine cerebral vasculature: a prospective, randomized, blinded assessment of safety and vessel response. J Neurosurg. 100:688–694. 2004.
Article10. Levy EI, Hanel RA, Tio FO, Garlick DS, Bailey L, Cunningham MR, et al. Safety and pharmacokinetics of sirolimus-eluting stents in the canine cerebral vasculature: 180 day assessment. Neurosurgery. 59:925–933. discussion 933-934. 2006.
Article11. Maramattom BV, Wijdicks EF. Sirolimus may not cause neurotoxicity in kidney and liver transplant recipients. Neurology. 63:1958–1959. 2004.
Article12. Park S, Lee DG, Chung WJ, Lee DH, Suh DC. Long-term outcomes of drug-eluting stents in symptomatic intracranial stenosis. Neurointervention. 8:9–14. 2013.
Article13. Qureshi AI, Kirmani JF, Hussein HM, Harris-Lane P, Divani AA, Suri MF, et al. Early and intermediate-term outcomes with drug-eluting stents in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 59:1044–1051. discussion 1051. 2006.
Article14. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 22:659–661. 2008.
Article15. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (taxol). Semin Oncol. 20(4 Suppl 3):1–15. 1993.16. Steinfort B, Ng PP, Faulder K, Harrington T, Grinnell V, Sorby W, et al. Midterm outcomes of paclitaxel-eluting stents for the treatment of intracranial posterior circulation stenoses. J Neurosurg. 106:222–225. 2007.
Article17. Velasco R, Bruna J. Taxane-induced peripheral neurotoxicity. Toxics. 3:152–169. 2015.
Article18. Waksman R, Pakala R. Drug-eluting balloon: the comeback kid? Circ Cardiovasc Interv. 2:352–358. 2009.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Non-convulsive seizure related to Cremophor ELâ„¢-free, polymeric micelle formulation of paclitaxel: a case report
- Characteristics of Focused Ultrasound Mediated Blood-Brain Barrier Opening in Magnetic Resonance Images
- Transient Blood-Brain Barrier Disruption Induced by Cerebral Concussion
- Regulation of paclitaxel-induced programmed cell death by autophagic induction: A model for cervical cancer
- Iron mediates endothelial cell damage and blood-brain barrier opening in the hippocampus after transient forebrain ischemia in rats