Obstet Gynecol Sci.
2018 May;61(3):421-424. 10.5468/ogs.2018.61.3.421.
Non-convulsive seizure related to Cremophor ELâ„¢-free, polymeric micelle formulation of paclitaxel: a case report
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. nwlee@korea.ac.kr
- KMID: 2416133
- DOI: http://doi.org/10.5468/ogs.2018.61.3.421
Abstract
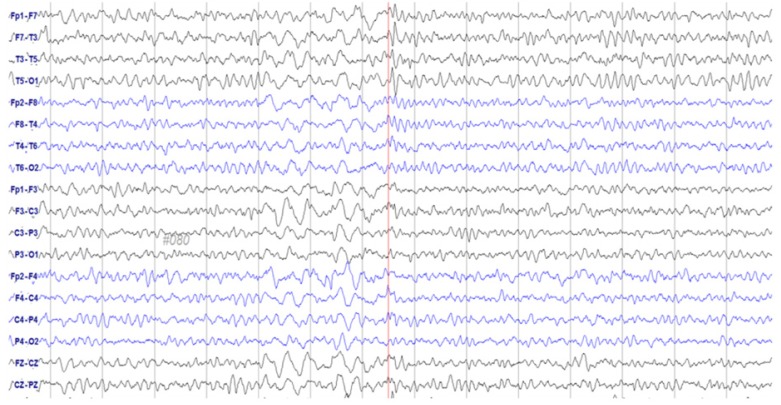
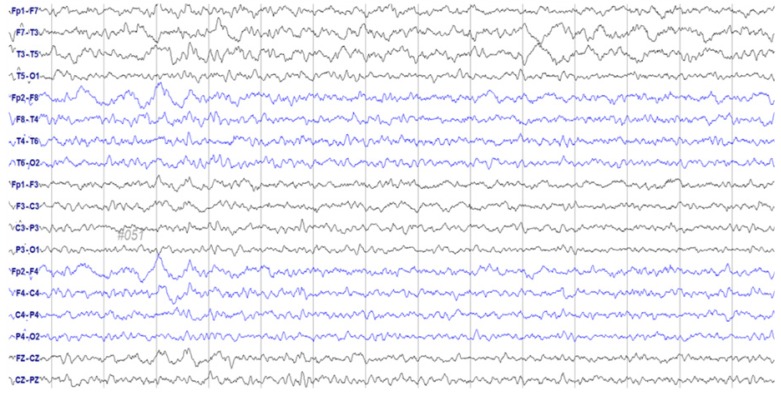
- Paclitaxel is a chemotherapeutic agent that is effective against ovarian, breast, lung, and other cancers. Although peripheral neurotoxicity is among the most common side effects of paclitaxel treatment, central neurotoxicity is rarely reported. When centrally mediated side effects are observed, they are attributed to Cremophor ELâ„¢ (CrEL), a surfactant-containing vehicle used for paclitaxel administration. In the present report, we discuss the case of a 72-year-old woman with ovarian carcinoma who experienced a non-convulsive seizure following administration of a CrEL-free, polymeric micelle formulation of paclitaxel. One week after her fourth round of chemotherapy, she experienced a transient episode of aphasia for 45 minutes. Electroencephalography demonstrated epileptiform discharges. To our knowledge, this is the first reported case of seizure associated with a CrEL-free formulation of paclitaxel. Although rare, patients and clinicians should remain aware of the risk of non-convulsive seizure following infusion of this paclitaxel formulation.
Keyword
MeSH Terms
Figure
Reference
-
1. Magge RS, DeAngelis LM. The double-edged sword: neurotoxicity of chemotherapy. Blood Rev. 2015; 29:93–100. PMID: 25445718.
Article2. Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001; 37:1590–1598. PMID: 11527683.3. Deepa G, Ashwanikumar N, Pillai JJ, Kumar GS. Polymer nanoparticles--a novel strategy for administration of Paclitaxel in cancer chemotherapy. Curr Med Chem. 2012; 19:6207–6213. PMID: 22834822.4. Illán-Gala I, Díaz de Terán FJ, Alonso P, Aguilar-Amat MJ. Nonconvulsive status epilepticus secondary to paclitaxel administration. Epilepsy Behav Case Rep. 2015; 4:20–22. PMID: 26106578.
Article5. O'Connor TL, Kossoff E. Delayed seizure associated with paclitaxel-Cremophor el in a patient with early-stage breast cancer. Pharmacotherapy. 2009; 29:993–996. PMID: 19637953.6. Muallaoğlu S, Koçer M, Güler N. Acute transient encephalopathy after weekly paclitaxel infusion. Med Oncol. 2012; 29:1297–1299. PMID: 21618057.
Article7. Beleza P, Rocha J, Pinho J. Diagnosis, etiology, and treatment of nonconvulsive status epilepticus, a semiological oriented review. Neurologist. 2015; 19:160–167. PMID: 26075471.
Article8. Lee SW, Kim YM, Kim YT, Kang SB. An open-label, multicenter, phase I trial of a cremophor-free, polymeric micelle formulation of paclitaxel combined with carboplatin as a first-line treatment for advanced ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG-3016). J Gynecol Oncol. 2016; 28:e26. PMID: 28028994.
Article9. Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004; 10:3708–3716. PMID: 15173077.
Article10. Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013; 63:419–437. PMID: 24590861.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Ischemic Colitis Associated with Paclitaxel Loaded Polymeric Micelle (Genexol-PM(R)) Chemotherapy
- An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer
- An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021)
- The Hidden Culprit: A Case of Repeated Anaphylaxis to Cremophor
- A Case of Convulsive Seizure Development Induced by Clozapine