Obstet Gynecol Sci.
2013 Mar;56(2):84-92. 10.5468/OGS.2013.56.2.84.
Regulation of paclitaxel-induced programmed cell death by autophagic induction: A model for cervical cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea College of Medicine, Seoul, Korea. hohoho@catholic.ac.kr
- 2Department of Obstetrics and Gynecology, Mahidol University, Bangkok, Thailand.
- KMID: 1425655
- DOI: http://doi.org/10.5468/OGS.2013.56.2.84
Abstract
OBJECTIVE
Autophagy plays a vital role in homeostasis by combining organelles and cellular proteins with lysosome under starvation conditions. In addition, autophagy provides tumor cells with a source of energy. Continued autophagy will induce cells death. Here we aim to see if autophagic induction has an effect on conventional chemotherapeutic agents.
METHODS
Rapamycin, or mammalian target of rapamycin and paclitaxel, apoptosis-inducing agents were used autophagy in HeLa cervical cancer cells.
RESULTS
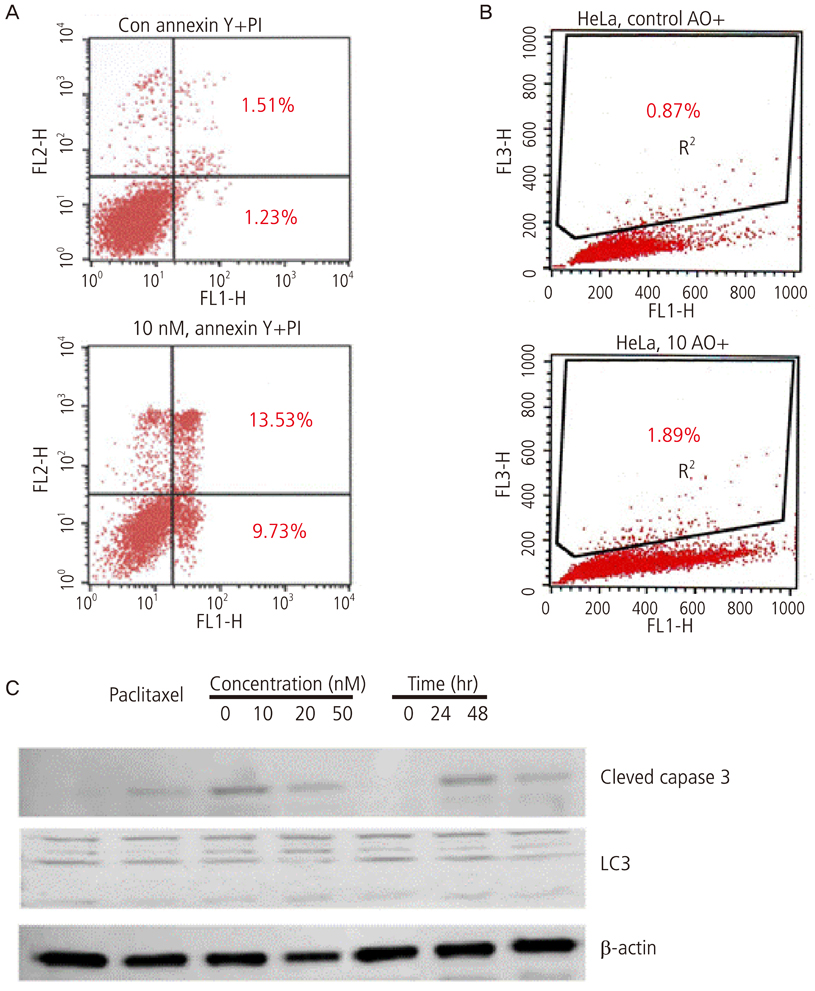
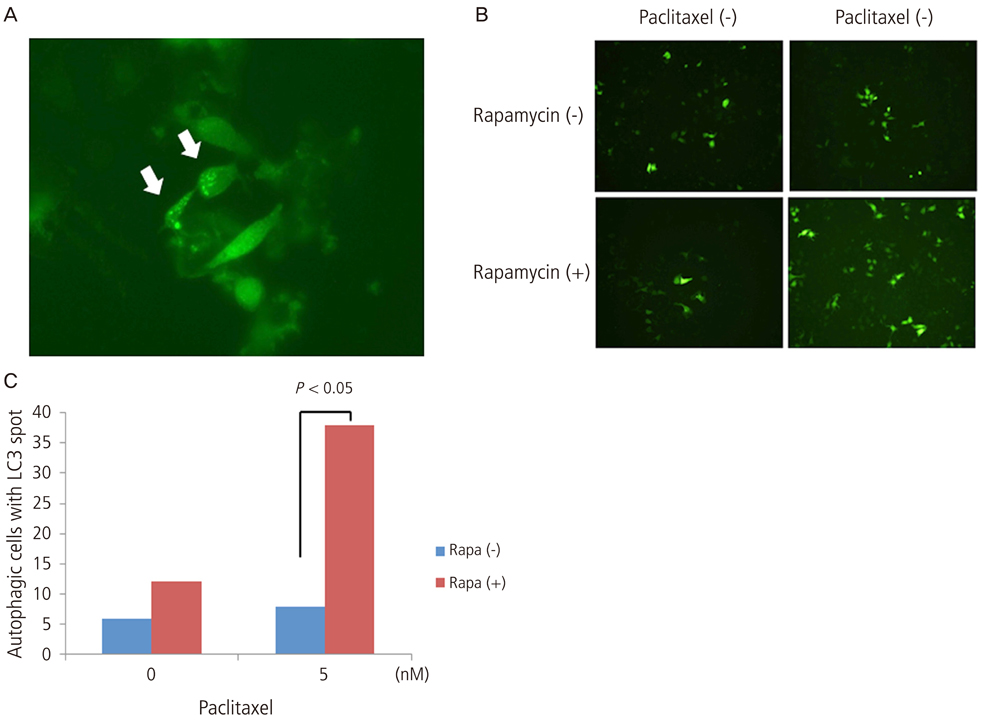
Growth inhibition of cells was not observed after the application of 0, 10, 20 nM of paclitaxel with or without rapamycin. Using a 5 nM concentration of paclitaxel, rapamycin administration inhibited cell growth significantly compared to no treatment. This implies the synergic antitumor effect of paclitaxel and rapamycin. Paclitaxel itself did not show any autophagic effect on cells but did show cell apoptosis by flow cytometry. Light chain 3, a microtubule-associated protein, which reflect autophagy, was increased with 5 nM of paclitaxel after pretreatment with 10 nM of rapamycin.
CONCLUSION
These findings suggest that the autophagic inducer, rapamycin, can potentiate autophagic cell death when added as an apoptosis-inducing chemotherapeutic agent. In conclusion, the control of autophagy may be a future target for chemotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005. 5:726–734.2. Kondo Y, Kondo S. Autophagy and cancer therapy. Autophagy. 2006. 2:85–90.3. Tewari KS, Monk BJ. Recent achievements and future developments in advanced and recurrent cervical cancer: trials of the Gynecologic Oncology Group. Semin Oncol. 2009. 36:170–180.4. Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999. 402:672–676.5. Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005. 122:927–939.6. Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003. 112:1809–1820.7. Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003. 100:15077–15082.8. Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010. 6:239–247.9. Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005. 102:8204–8209.10. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011. 469:323–335.11. Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004. 304:1500–1502.12. Ravikumar B, Berger Z, Vacher C, O'Kane CJ, Rubinsztein DC. Rapamycin pre-treatment protects against apoptosis. Hum Mol Genet. 2006. 15:1209–1216.13. Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol. 2009. 20:1–7.14. Pectasides D, Kamposioras K, Papaxoinis G, Pectasides E. Chemotherapy for recurrent cervical cancer. Cancer Treat Rev. 2008. 34:603–613.15. del Campo JM, Prat A, Gil-Moreno A, Perez J, Parera M. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008. 110:S72–S76.16. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011. 12:21–35.17. Lee SB, Tong SY, Kim JJ, Um SJ, Park JS. Caspase-independent autophagic cytotoxicity in etoposide-treated CaSki cervical carcinoma cells. DNA Cell Biol. 2007. 26:713–720.18. Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010. 294:204–210.19. Zou CF, Jia L, Jin H, Yao M, Zhao N, Huan J, et al. Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. BMC Cancer. 2011. 11:22.20. Xi G, Hu X, Wu B, Jiang H, Young CY, Pang Y, et al. Autophagy inhibition promotes paclitaxel-induced apoptosis in cancer cells. Cancer Lett. 2011. 307:141–148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- A Case of Nivolumab-induced Cutaneous Toxicity
- The mechanism of cell death in etoposide treated cervical cancer cells: apoptotic and non-apoptotic programmed cell death
- Clusterin expression and paclitaxel resistance in cervical cancer cell lines
- Paclitaxel-resistant cancer cell-derived secretomes elicit ABCB1-associated docetaxel cross-resistance and escape from apoptosis through FOXO3a-driven glycolytic regulation