J Korean Med Sci.
2022 Feb;37(7):e53. 10.3346/jkms.2022.37.e53.
Sodium-Glucose Cotransporter-2 Inhibitor-Related Diabetic Ketoacidosis: Accuracy Verification of Operational Definition
- Affiliations
-
- 1Drug Safety Monitoring Center, Seoul National University Hospital, Seoul, Korea
- 2College of Pharmacy, Sookmyung Women’s University, Seoul, Korea
- 3Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Division of Allergy and Clinical Immunology, Departmemt of Internal Medicine, Chungbuk National University Hospital, Chungbuk National College of Medicine, Cheongju, Korea
- 5Department of Pathology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 6Department of Pulmonology and Allergy, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 7Department of Digital Health, SAIHST, Sungkyunkwan University, Seoul, Korea
- 8Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2526516
- DOI: http://doi.org/10.3346/jkms.2022.37.e53
Abstract
- Background
The most important aspect of a retrospective cohort study is the operational definition (OP) of the disease. We developed a detailed OP for the detection of sodiumglucose cotransporter-2 inhibitors (SGLT2i) related to diabetic ketoacidosis (DKA). The OP was systemically verified and analyzed.
Methods
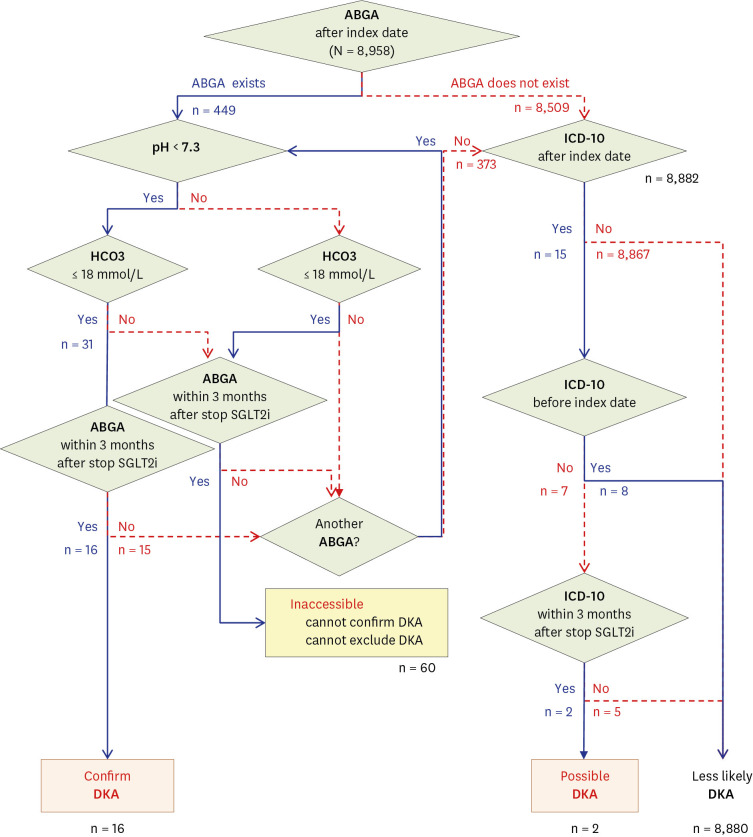
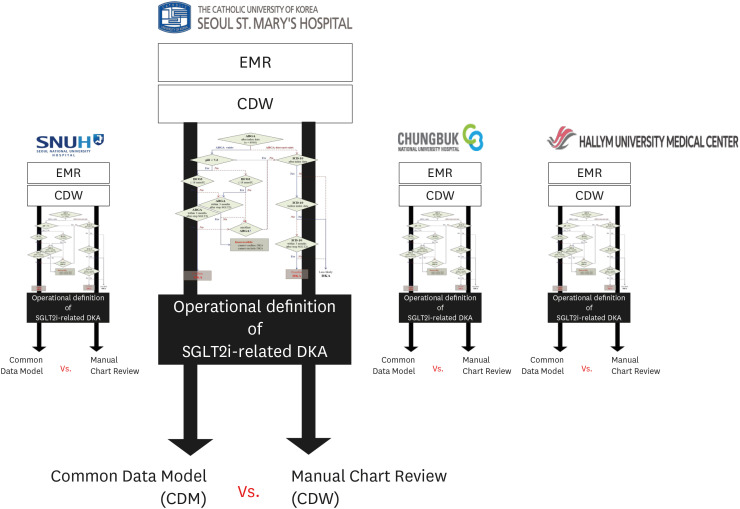
All patients prescribed SGLT2i at four university hospitals were enrolled in this experiment. A DKA diagnostic algorithm was created and distributed to each hospital; subsequently, the number of SGLT2i-related DKAs was confirmed. Then, the algorithm functionality was verified through manual chart reviews by an endocrinologist using the same OP.
Results
A total of 8,958 patients were initially prescribed SGLT2i. According to the algorithm, 0.18% (16/8,958) were confirmed to have SGLT2i-related DKA. However, based on manual chart reviews of these 16 cases, there was only one case of SGLT2i-related DKA (positive predictive value = 6.3%). Even after repeatedly narrowing the diagnosis range of the algorithm, the effect of a positive predictive value was insignificant (6.3–10.0%, P > 0.999).
Conclusion
Owing to the nature of electronic medical record data, we could not create an algorithm that clearly differentiates SGLT2i-related DKA despite repeated attempts. In all retrospective studies, a portion of the samples should be randomly selected to confirm the accuracy of the OP through chart review. In retrospective cohort studies in which chart review is not possible, it will be difficult to guarantee the reliability of the results.
Keyword
Figure
Cited by 2 articles
-
Long-Term Changes in HbA1c According to Blood Glucose Control Status During the First 3 Months After Visiting a Tertiary University Hospital
Hyunah Kim, Da Young Jung, Seung-Hwan Lee, Jae-Hyoung Cho, Hyeon Woo Yim, Hun-Sung Kim
J Korean Med Sci. 2022;37(38):e281. doi: 10.3346/jkms.2022.37.e281.Real-World Treatment Intensity and Patterns in Patients With Myopic Choroidal Neovascularization: Common Data Model in Ophthalmology
Manh-Hung Bui, Da Yun Lee, Sang Jun Park, Kyu Hyung Park
J Korean Med Sci. 2023;38(23):e174. doi: 10.3346/jkms.2023.38.e174.
Reference
-
1. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S90–102. PMID: 30559235.2. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43(4):398–406. PMID: 31441247.
Article3. Esteban-Jiménez O, Navarro-Pemán C, Urieta-González L. Safety of SGLT2 inhibitors. A review of the adverse drug reactions registered in a national database. Semergen. 2018; 44(1):23–29. PMID: 29183654.4. Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. 2019; 63:9–14. PMID: 30910328.
Article5. Fadini GP, Bonora BM, Avogaro A. SGLT2 inhibitors and diabetic ketoacidosis: data from the FDA Adverse Event Reporting System. Diabetologia. 2017; 60(8):1385–1389. PMID: 28500396.
Article6. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017; 37(2):187–194. PMID: 27931088.
Article7. Bonora BM, Avogaro A, Fadini GP. Sodium-glucose co-transporter-2 inhibitors and diabetic ketoacidosis: an updated review of the literature. Diabetes Obes Metab. 2018; 20(1):25–33. PMID: 28517913.
Article8. Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018; 33(34):e213. PMID: 30127705.
Article9. Kim HS, Kim JH. Proceed with caution when using real world data and real world evidence. J Korean Med Sci. 2019; 34(4):e28. PMID: 30686950.
Article10. Lai EC, Ryan P, Zhang Y, Schuemie M, Hardy NC, Kamijima Y, et al. Applying a common data model to Asian databases for multinational pharmacoepidemiologic studies: opportunities and challenges. Clin Epidemiol. 2018; 10:875–885. PMID: 30100761.
Article11. Kim HS, Kim DJ, Yoon KH. Medical big data is not yet available: why we need realism rather than exaggeration. Endocrinol Metab (Seoul). 2019; 34(4):349–354. PMID: 31884734.
Article12. Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012; 19(1):54–60. PMID: 22037893.
Article13. Nyenwe EA, Kitabchi AE. The evolution of diabetic ketoacidosis: an update of its etiology, pathogenesis and management. Metabolism. 2016; 65(4):507–521. PMID: 26975543.
Article14. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may oredispose to ketoacidosis. J Clin Endocrinol Metab. 2015; 100(8):2849–2852. PMID: 26086329.15. Singh AK. Sodium-glucose co-transporter-2 inhibitors and euglycemic ketoacidosis: Wisdom of hindsight. Indian J Endocrinol Metab. 2015; 19(6):722–730. PMID: 26693421.
Article16. Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016; 12(4):222–232. PMID: 26893262.
Article17. Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015; 38(9):1680–1686. PMID: 26203064.
Article18. Tang H, Li D, Wang T, Zhai S, Song Y. Effect of sodium-glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2016; 39(8):e123–e124. PMID: 27311492.
Article19. Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013; 27(5):473–478. PMID: 23849632.
Article20. Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: a review. Curr Diabetes Rev. 2017; 13(3):315–321. PMID: 27097605.
Article21. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009; 32(7):1335–1343. PMID: 19564476.
Article22. Laakso M, Pyörälä K. Age of onset and type of diabetes. Diabetes Care. 1985; 8(2):114–117. PMID: 3873328.
Article23. Goldenberg RM, Berard LD, Cheng AY, Gilbert JD, Verma S, Woo VC, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016; 38(12):2654–2664.e1. PMID: 28003053.
Article24. Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, et al. Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet. J Diabetes Investig. 2015; 6(5):587–590.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Euglycemic Diabetic Ketoacidosis When Reducing Insulin Dosage in Patients Taking Sodium Glucose Cotransporter 2 Inhibitor
- SGLT2 Inhibitors and Ketoacidosis: Pathophysiology and Management
- Sodium-Glucose Cotransporter 2 Inhibitors for People with Type 1 Diabetes
- A Case of Diabetic Ketoacidosis Induced by Sodium-Glucose Cotransporter 2 Inhibitor
- Euglycemic diabetic ketoacidosis development in a patient with type 2 diabetes receiving a sodium-glucose cotransporter-2 inhibitor and a carbohydrate-restricted diet