Clin Endosc.
2022 Jan;55(1):101-112. 10.5946/ce.2021.066.
Colorectal Cancer Screening with Computed Tomography Colonography: Single Region Experience in Kazakhstan
- Affiliations
-
- 1Department of Radiology and Nuclear Medicine, Kazakh Institute of Oncology and Radiology, Almaty, Kazakhstan
- 2Department of Visual Diagnostics, Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan
- 3Department of Medical Oncology, Kazakh Institute of Oncology and Radiology, Almaty, Kazakhstan
- 4Department of Radiology, Medical College at the University of Kentucky, Lexington, KY, USA
- 5Center of Morphological Diagnostics, Kazakh Institute of Oncology and Radiology, Almaty, Kazakhstan
- 6Department of Endoscopy, Kazakh Institute of Oncology and Radiology, Almaty, Kazakhstan
- KMID: 2525056
- DOI: http://doi.org/10.5946/ce.2021.066
Abstract
- Background/Aims
The aim of our study was to determine the efficacy of computed tomography colonography (CTC) in screening for colorectal cancer (CRC).
Methods
A total of 612 females and 588 males aged 45 to 75 years were enrolled in CTC screening. CTC was performed following standard bowel preparation and colonic insufflation with carbon dioxide. The main outcomes were the detection rate of CRC and advanced adenoma (AA), prevalence of colorectal lesions in relation to socio-demographic and health factors, and overall diagnostic performance of CTC.
Results
Overall, 56.5% of the 1,200 invited subjects underwent CTC screening. The sensitivity for CRC and AA was 0.89 and 0.97, respectively, while the specificity was 0.71 and 0.99, respectively. The prevalence of CRC and AA was 3.0% (18/593) and 7.1% (42/593), respectively, with the highest CRC prevalence in the 66-75 age group (≥12 times; odds ratio [OR], 12.11; 95% confidence interval [CI], 4.45-32.92). CRC and AA prevalence were inversely correlated with Asian descent, physical activity, and negative fecal immunochemical test results (OR=0.43; 95% CI, 0.22-0.83; OR=0.16; 95% CI, 0.04-0.68; OR=0.5; 95% CI, 0.07-3.85, respectively).
Conclusions
Our study revealed high accuracy of CTC in diagnosing colonic neoplasms, good compliance with CTC screening, and high detection rate of CRC.
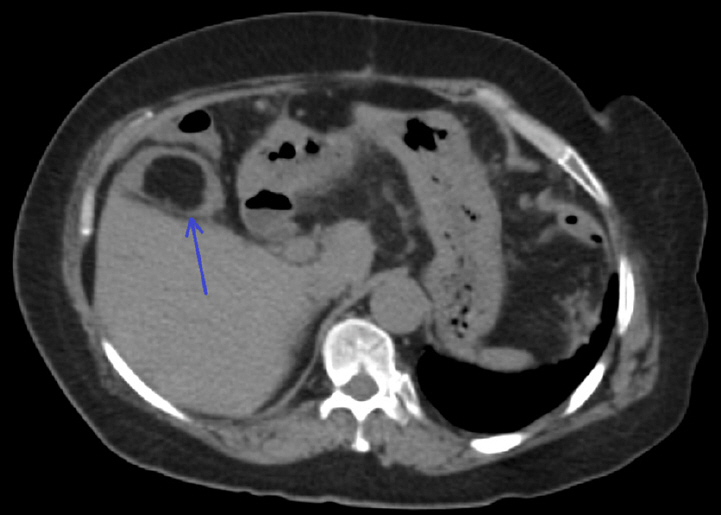
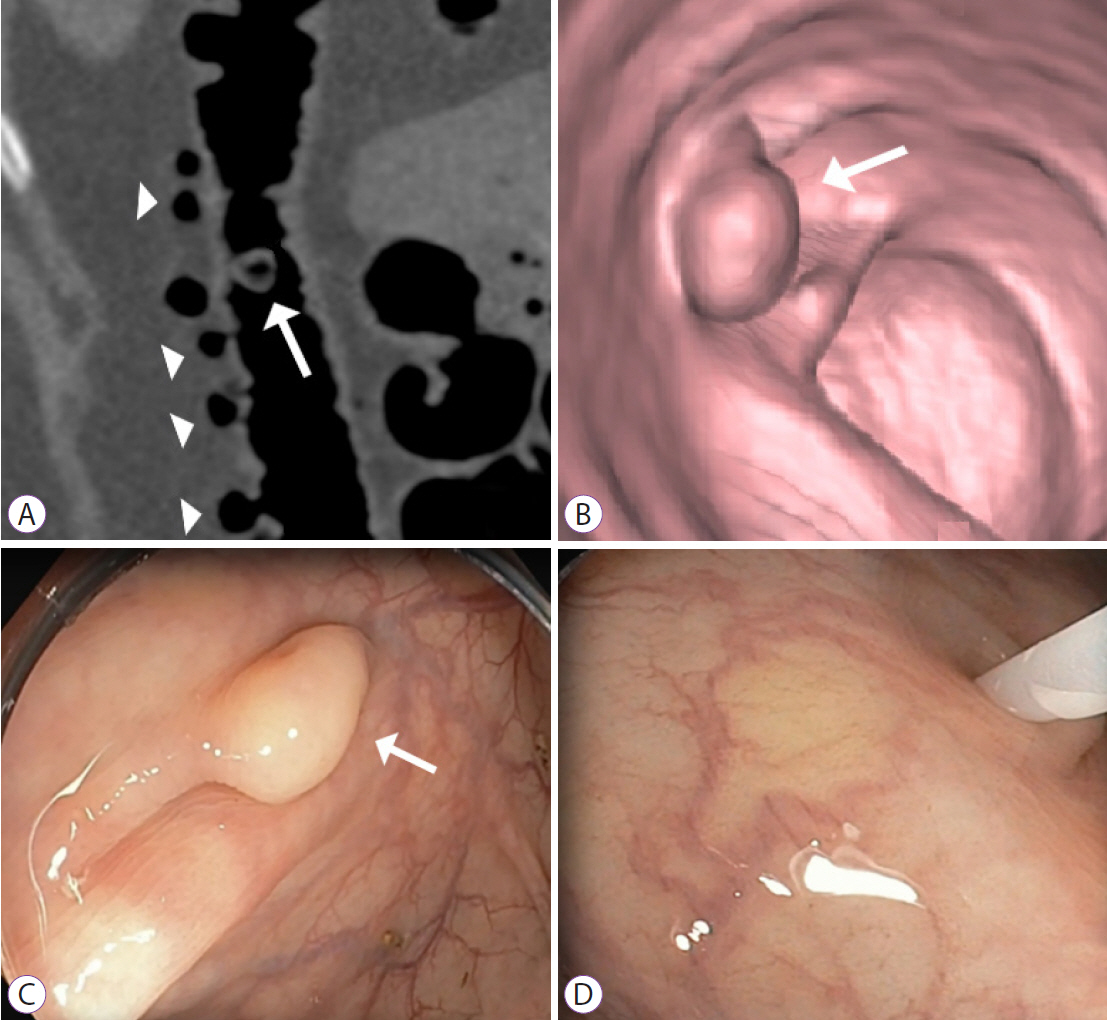
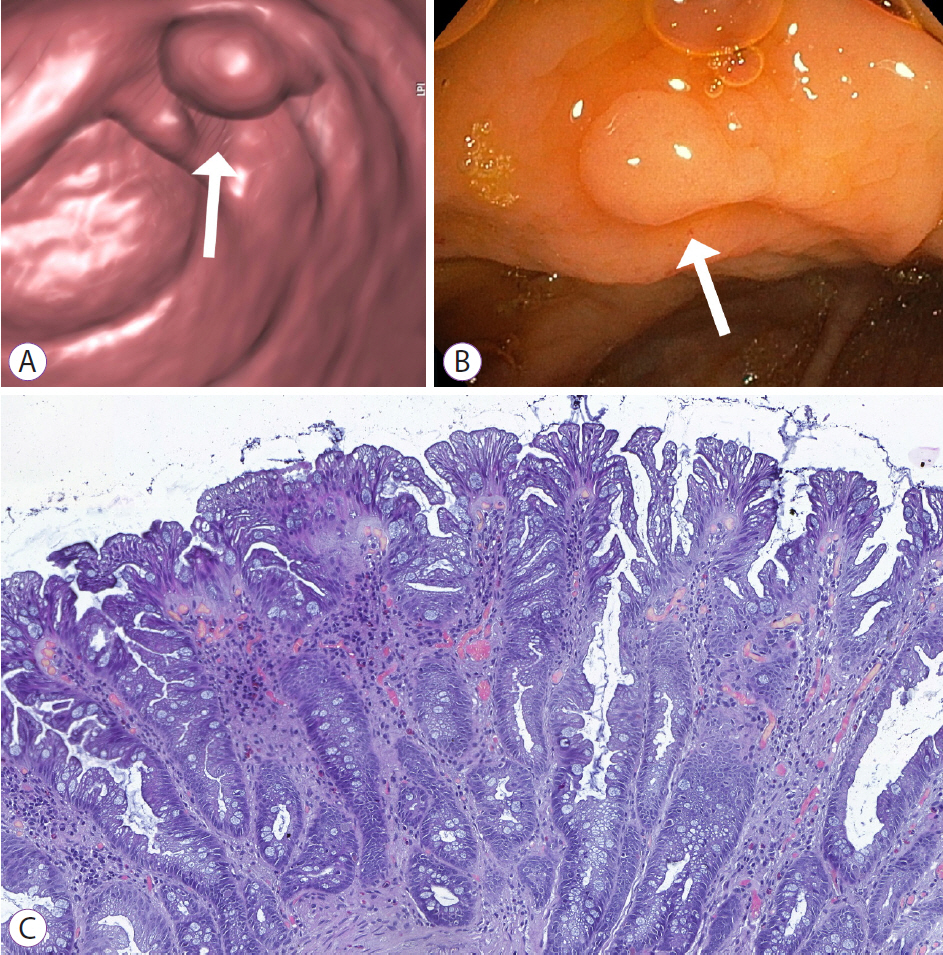
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.2. Pourhoseingholi MA. Increased burden of colorectal cancer in Asia. World J Gastrointest Oncol. 2012; 4:68–70.3. Zhylkaidarova A, Kaidarova D, Batyrbekov K, Shatkovskaya O, Begimbetova D. Trends of colorectal cancer prevalence in kazakhstan related to screening. Clin Endosc. 2021; 54:32–37.4. Okugawa Y, Grady WM, Goel A. Epigenetic alterations in colorectal cancer: Emerging Biomarkers. Gastroenterology. 2015; 149:1204–1225.e12.5. Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012; 107:1315–1329.6. Kahi CJ. Reviewing the evidence that polypectomy prevents cancer. Gastrointest Endosc Clin N Am. 2019; 29:577–585.7. Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the us multi-society task force on colorectal cancer. Gastroenterology. 2020; 158:1131–1153.e5.8. Sung JJY, Ng SC, Chan FKL, et al. An updated asia pacific consensus recommendations on colorectal cancer screening. Gut. 2015; 64:121–132.9. Ojidu H, Palmer H, Lewandowski J, et al. Patient tolerance and acceptance of different colonic imaging modalities: an observational cohort study. Eur J Gastroenterol Hepatol. 2018; 30:520–525.10. Abu-Freha N. [Should colonoscopy be the primary screening modality for colorectal cancer in israel?]. Harefuah. 2019; 158:523–528.11. Kaidarova D, Zhylkaidarova A, Jumanov A, Shatkovskaya O, Mukhametbek B. Colorectal screening in kazakhstan: analysis of accessibility, problems, and prospects for further improvement. Oncol and Rad of Kazakhstan. 2019; 1:4–8.12. Plumb AA, Halligan S, Pendsé DA, Taylor SA, Mallett S. Sensitivity and specificity of CT colonography for the detection of colonic neoplasia after positive faecal occult blood testing: systematic review and meta-analysis. Eur Radiol. 2014; 24:1049–1058.13. Neri E, Faggioni L, Cerri F, et al. CT colonography versus double-contrast barium enema for screening of colorectal cancer: comparison of radiation burden. Abdom Imaging. 2010; 35:596–601.14. Pickhardt PJ. Strong evidence in support of CT colonography screening. Lancet Oncol. 2012; 13:6–7.15. Atkin W, Dadswell E, Wooldrage K, et al. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013; 381:1194–1202.16. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: us preventive services task force recommendation statement. JAMA. 2016; 315:2564–2575.17. Spada C, Hassan C, Bellini D, et al. Imaging alternatives to colonoscopy: CT colonography and colon capsule. European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline - Update 2020. Endoscopy. 2020; 52:1127–1141.18. Sali L, Regge D. CT colonography for population screening of colorectal cancer: hints from European trials. Br J Radiol. 2016; 89:20160517.19. Sali L, Grazzini G, Ventura L, et al. Computed tomographic colonography in subjects with positive faecal occult blood test refusing optical colonoscopy. Dig Liver Dis. 2013; 45:285–289.20. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020; 76:182–188.21. Salmo E, Haboubi N. Adenoma and malignant colorectal polyp: pathological considerations and clinical applications. EMJ Gastroenterol. 2018; 7:92–102.22. Pooler BD, Kim DH, Lam VP, Burnside ES, Pickhardt PJ. CT colonography reporting and data system (C-RADS): benchmark values from a clinical screening program. AJR Am J Roentgenol. 2014; 202:1232–1237.23. Zhu H, Li F, Tao K, et al. Comparison of the participation rate between CT colonography and colonoscopy in screening population: a systematic review and meta-analysis of randomized controlled trials. Br J Radiol. 2020; 93:20190240.24. McNamara D, Qasim A, Lee N, Condon C, O’Morain C. Round one of the adelaide and meath hospital/trinity college colorectal cancer screening programme: programme report and analysis based on established international key performance indices. Ir J Med Sci. 2011; 180:549–552.25. Poskus T, Strupas K, Mikalauskas S, et al. Initial results of the national colorectal cancer screening program in Lithuania. Eur J Cancer Prev. 2015; 24:76–80.26. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015; 81:31–53.27. Amano T, Nishida T, Shimakoshi H, et al. Number of polyps detected is a useful indicator of quality of clinical colonoscopy. Endosc Int Open. 2018; 6:E878–E884.28. Klair JS, Ashat M, Johnson D, et al. Serrated polyp detection rate and advanced adenoma detection rate from a US multicenter cohort. Endoscopy. 2020; 52:61–67.29. Karsenti D, Tharsis G, Burtin P, et al. Adenoma and advanced neoplasia detection rates increase from 45 years of age. World J Gastroenterol. 2019; 25:447–456.30. Armaroli P, Villain P, Suonio E, et al. European code against cancer, 4th edition: cancer screening. Cancer Epidemiol. 2015; 39(Suppl 1):S139–S152.31. Abdullayev MS, Nurgaziyev KS, Zhylkaydarova AZ, Mansurova AB. Epidemiological aspects of colorectal cancer in Kazakhstan. Nov Khirurgii. 2017; 25:394–403.32. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.33. Kim NH, Jung YS, Lim JW, Park JH, Park DI, Sohn CI. Yield of repeat colonoscopy in asymptomatic individuals with a positive fecal immunochemical test and recent colonoscopy. Gastrointest Endosc. 2019; 89:1037–1043.34. Zhou XY, Yan L, Wang LL, Wang J. Association between physical activity and colorectal cancer risk and prognosis: A meta-analysis. Cancer Treatment and Research Communications. 2016; 9:62–69.35. Des Guetz G, Uzzan B, Bouillet T, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract. 2013; 2013:340851.36. Veerappan GR, Ally MR, Choi J-HR, Pak JS, Maydonovitch C, Wong RKH. Extracolonic findings on CT colonography increases yield of colorectal cancer screening. AJR Am J Roentgenol. 2010; 195:677–686.37. Pooler BD, Kim DH, Pickhardt PJ. Indeterminate but likely unimportant extracolonic findings at screening CT Colonography (C-RADS Category E3): incidence and outcomes data from a clinical screening program. AJR Am J Roentgenol. 2016; 207:996–1001.38. Tutein Nolthenius CJ, Boellaard TN, de Haan MC, et al. Computer tomography colonography participation and yield in patients under surveillance for 6-9 mm polyps in a population-based screening trial. Eur Radiol. 2016; 26:2762–2770.39. Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) consortium. Ann Intern Med. 2019; 171:756–764.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can Computed Tomography Colonography Replace Optical Colonoscopy in Detecting Colorectal Lesions?: State of the Art
- Early Detection of and Screening for Colorectal Neoplasia
- A Comparison of Patient Acceptance and Preferences Between CT Colonography and Conventional Colonoscopy in Colorectal Cancer Screening
- Trends of Colorectal Cancer Prevalence in Kazakhstan Related to Screening
- CT Colonography