Ann Surg Treat Res.
2021 Nov;101(5):266-273. 10.4174/astr.2021.101.5.266.
Limits of serum carcinoembryonic antigen and carbohydrate antigen 19-9 as the diagnosis of gallbladder cancer
- Affiliations
-
- 1Department of Surgery and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2521848
- DOI: http://doi.org/10.4174/astr.2021.101.5.266
Abstract
- Purpose
Although serum CEA and CA 19-9 have been widely utilized for the diagnosis of gallbladder cancer (GBC), few studies have examined the diagnostic performance of them. This study aimed to investigate the diagnostic performance of these 2 biomarkers and demonstrate their clinical usefulness in diagnosing GBC.
Methods
Between January 2000 and March 2020, a total of 751 GBC patients and 2,310 normal controls were included. Serum CEA and CA 19-9 were measured preoperatively. Receiver operating characteristic curves were obtained, and the sensitivity and specificity of each biomarker were evaluated.
Results
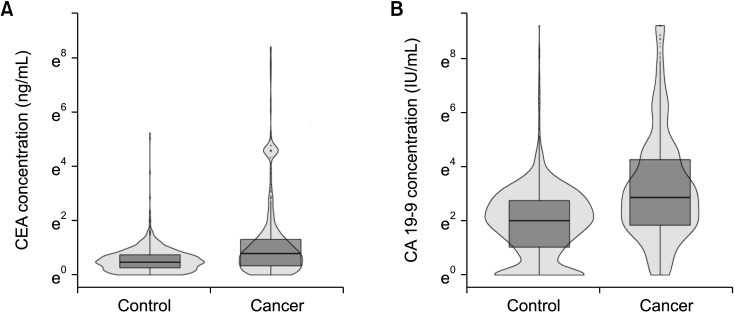
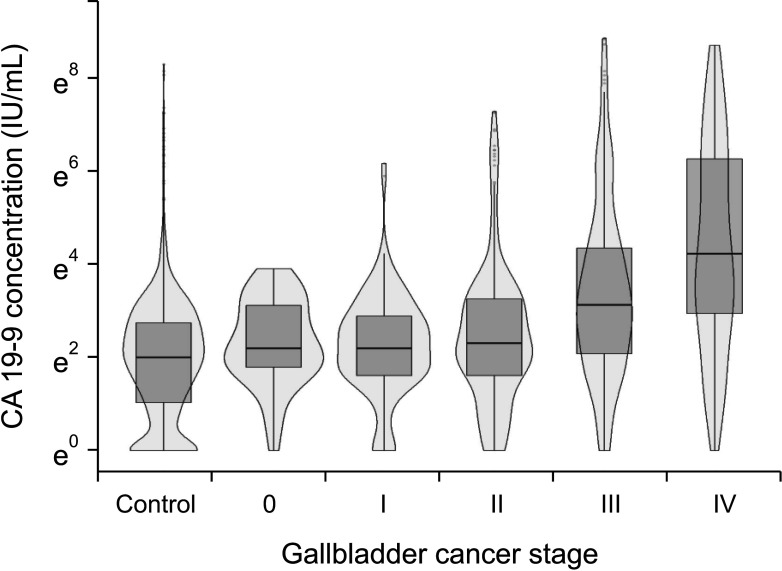
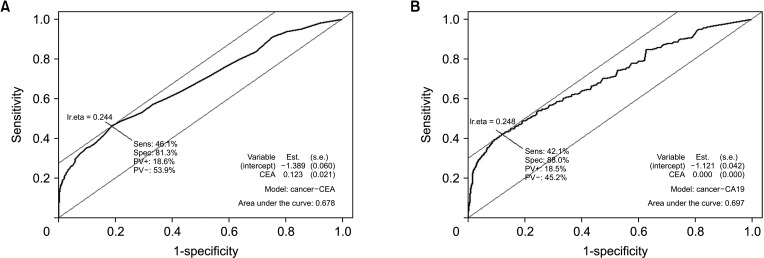
In terms of differentiating GBC from the control, the sensitivity and specificity of serum CEA at 5 ng/mL was 12.1% and 99.1%, respectively, and those of serum CA 19-9 at 37 IU/mL were 28.7% and 94.5%, respectively. The optimal cutoff values of CEA and CA 19-9 were set to 2.1 ng/mL and 26 IU/mL in the receiver operating characteristic curves, respectively. The sensitivities of CEA and CA 19-9 at new cutoff values slightly increased but remained low (CEA, 42.9%; CA 19-9, 38.2%). When differentiating early-stage GBC from advanced tumor, the sensitivity and specificity, were 14.2% and 96.1% for CEA (cutoff value, 5 ng/mL) and 33.6% and 90.1% for CA 19-9 (cutoff value, 37 IU/mL), respectively.
Conclusion
Serum CEA and CA 19-9 levels are not suitable for screening GBC patients from controls. New promising biomarkers with higher sensitivity should be explored.
Figure
Reference
-
1. Rakić M, Patrlj L, Kopljar M, Kliček R, Kolovrat M, Loncar B, et al. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014; 3:221–226. PMID: 25392833.2. Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010; 15:168–181. PMID: 20147507.
Article3. Kumar S, Bhoriwal S, Muduly D, Kar M, Sharma A, Pathy S, et al. Multimodality management of incidentally detected gall bladder cancer: long term results from a tertiary care cancer centre. J Gastrointest Oncol. 2019; 10:128–133. PMID: 30788168.
Article4. Mamdani H, Ahmed S, Armstrong S, Mok T, Jalal SI. Blood-based tumor biomarkers in lung cancer for detection and treatment. Transl Lung Cancer Res. 2017; 6:648–660. PMID: 29218268.
Article5. Kau SY, Shyr YM, Su CH, Wu CW, Lui WY. Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J Am Coll Surg. 1999; 188:415–420. PMID: 10195726.6. Björnsson E, Kilander A, Olsson R. CA 19-9 and CEA are unreliable markers for cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver. 1999; 19:501–508. PMID: 10661684.7. Singh S, Tang SJ, Sreenarasimhaiah J, Lara LF, Siddiqui A. The clinical utility and limitations of serum carbohydrate antigen (CA19-9) as a diagnostic tool for pancreatic cancer and cholangiocarcinoma. Dig Dis Sci. 2011; 56:2491–2496. PMID: 21516323.
Article8. Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012; 3:105–119. PMID: 22811878.9. Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001; 93:1054–1061. PMID: 11459866.
Article10. Muinao T, Deka Boruah HP, Pal M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon. 2019; 5:e02826. PMID: 31867451.
Article11. Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine (Baltimore). 2017; 96:e5811. PMID: 28296720.
Article12. Chapman MH, Sandanayake NS, Andreola F, Dhar DK, Webster GJ, Dooley JS, et al. Circulating CYFRA 21-1 is a specific diagnostic and prognostic biomarker in biliary tract cancer. J Clin Exp Hepatol. 2011; 1:6–12. PMID: 22228935.
Article13. Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009; 9:30. PMID: 19405942.
Article14. Loosen SH, Roderburg C, Kauertz KL, Pombeiro I, Leyh C, Benz F, et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. J Hepatol. 2017; 67:749–757. PMID: 28668580.
Article15. Macias RI, Kornek M, Rodrigues PM, Paiva NA, Castro RE, Urban S, et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019; 39 Suppl 1:108–122. PMID: 30843325.
Article16. Grunnet M, Mau-Sørensen M. Serum tumor markers in bile duct cancer: a review. Biomarkers. 2014; 19:437–443. PMID: 24857368.17. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
Article18. Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004; 19:182–186. PMID: 14731128.
Article19. Distler M, Pilarsky E, Kersting S, Grützmann R. Preoperative CEA and CA 19-9 are prognostic markers for survival after curative resection for ductal adenocarcinoma of the pancreas: a retrospective tumor marker prognostic study. Int J Surg. 2013; 11:1067–1072. PMID: 24161419.20. Duraker N, Hot S, Polat Y, Höbek A, Gençler N, Urhan N. CEA, CA 19-9, and CA 125 in the differential diagnosis of benign and malignant pancreatic diseases with or without jaundice. J Surg Oncol. 2007; 95:142–147. PMID: 17262731.
Article21. Berger AC, Garcia M Jr, Hoffman JP, Regine WF, Abrams RA, Safran H, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008; 26:5918–5922. PMID: 19029412.
Article22. Gooneti l leke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007; 33:266–270. PMID: 17097848.23. Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer. 2012; 76:138–143. PMID: 22153832.
Article24. Vukobrat-Bijedic Z, Husic-Selimovic A, Sofic A, Bijedic N, Bjelogrlic I, Gogov B, et al. Cancer antigens (CEA and CA 19-9) as markers of advanced stage of colorectal carcinoma. Med Arch. 2013; 67:397–401. PMID: 25568506.25. Xing H, Wang J, Wang Y, Tong M, Hu H, Huang C, et al. Diagnostic value of CA 19-9 and carcinoembryonic antigen for pancreatic cancer: a meta-analysis. Gastroenterol Res Pract. 2018; 2018:8704751. PMID: 30584422.
Article26. Jeong Y, Kim JH, Chae HD, Park SJ, Bae JS, Joo I, et al. Deep learning-based decision support system for the diagnosis of neoplastic gallbladder polyps on ultrasonography: preliminary results. Sci Rep. 2020; 10:7700. PMID: 32382062.
Article27. Reid KM, Ramos-De la Medina A, Donohue JH. Diagnosis and surgical management of gallbladder cancer: a review. J Gastrointest Surg. 2007; 11:671–681. PMID: 17468929.
Article28. Byun Y, Choi YJ, Kang JS, Han Y, Kim H, Kwon W, et al. Early outcomes of robotic extended cholecystectomy for the treatment of gallbladder cancer. J Hepatobiliary Pancreat Sci. 2020; 27:324–330. PMID: 32062866.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Individualized Cutoff Value of the Serum Carcinoembryonic Antigen Level According to TNM Stage in Colorectal Cancer
- Serum Carcinoembryonic Antigen for Recurrence in Colorectal Cancer Patients
- Changes of serum carcinoembryonic antigen in patients with colorectal cancer
- Comparison and correlation of carcinoembryonic antigen levels betwwen peripheral blood and inferior mesenteric vein blood, and gallbladder bile, and rectal secretion
- Assessment of prognostic role od serum carcinoembryonic antigen monitoring in patients with stomach, colorectal and breast cancers