Korean J Physiol Pharmacol.
2021 Nov;25(6):593-601. 10.4196/kjpp.2021.25.6.593.
Hydrogen sulfide, a gaseous signaling molecule, elongates primary cilia on kidney tubular epithelial cells by activating extracellular signal-regulated kinase
- Affiliations
-
- 1Department of Biotechnology, College of Fisheries Sciences, Pukyong National University, Busan 48513, Korea.
- 2Department of Molecular Medicine, Keimyung University School of Medicine, Daegu 42601, Korea.
- 3Department of Medicine, Medical University of South Carolina, SC 29425, USA.
- 4Department of Medicine, Ralph H. Johnson Veterans Affairs Medical Center, Charleston, SC 29425, USA.
- 5Department of Anatomy, BK21 Plus, Cardiovascular Research Institute, School of Medicine, Kyungpook National University, Daegu 41944, Korea.
- KMID: 2521485
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.593
Abstract
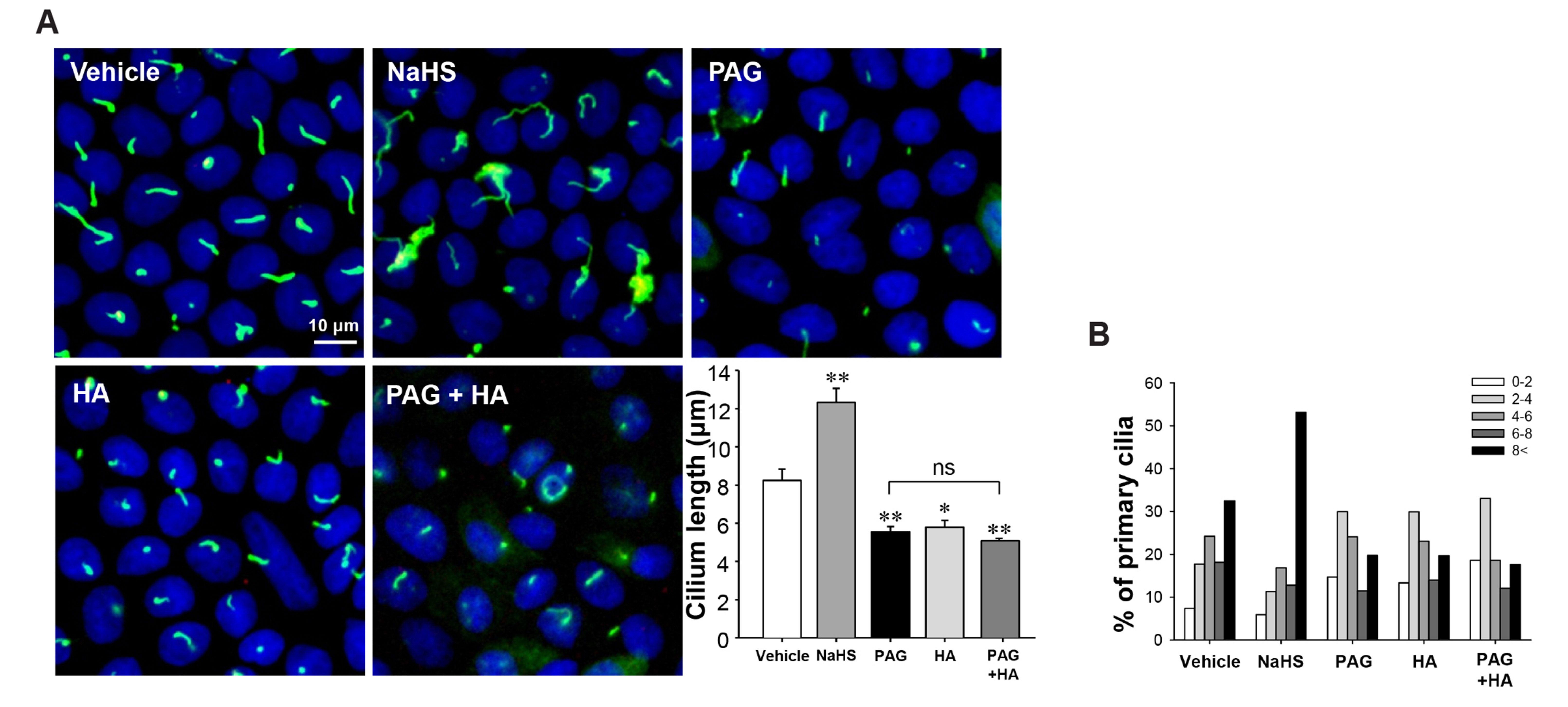
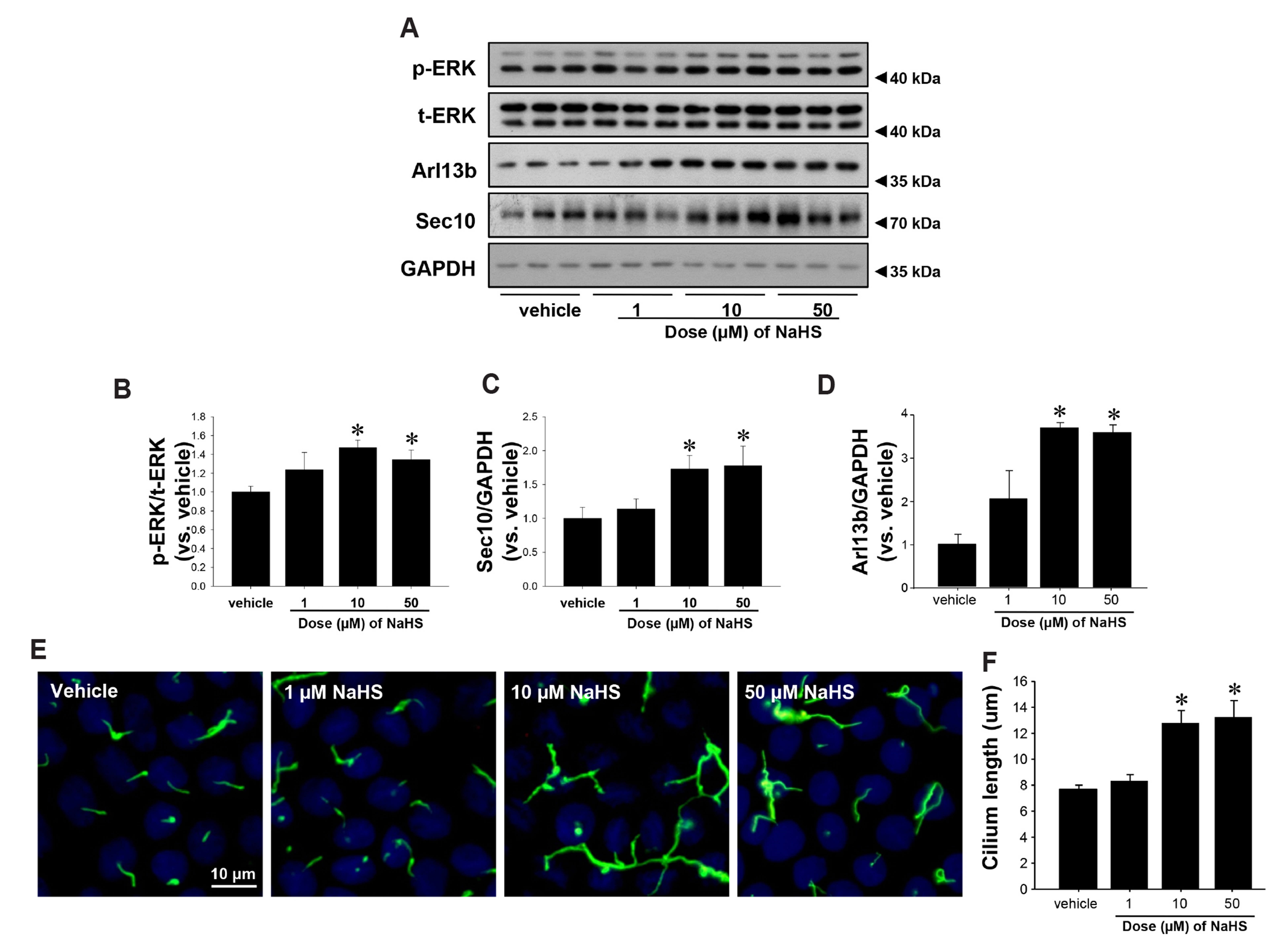
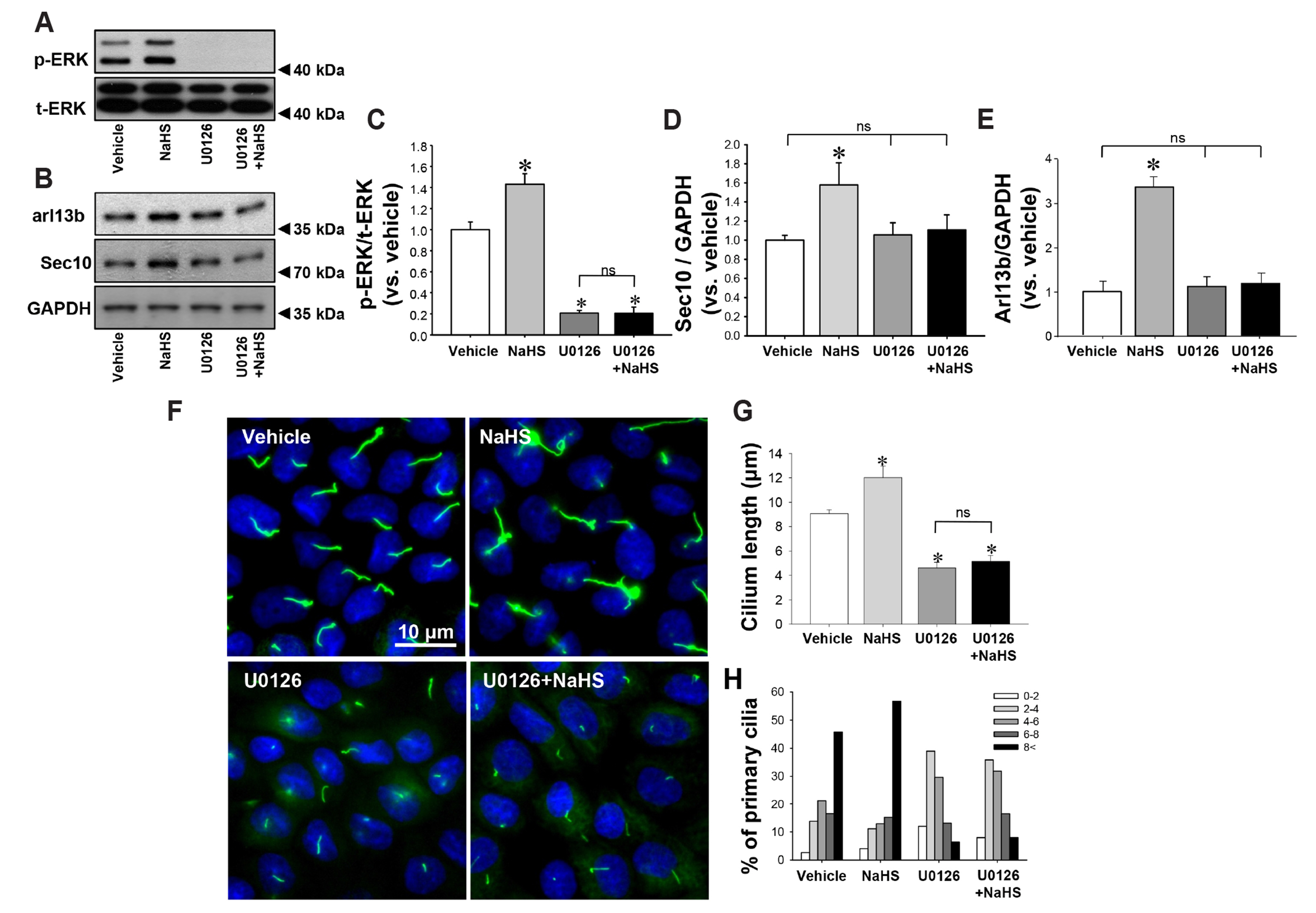
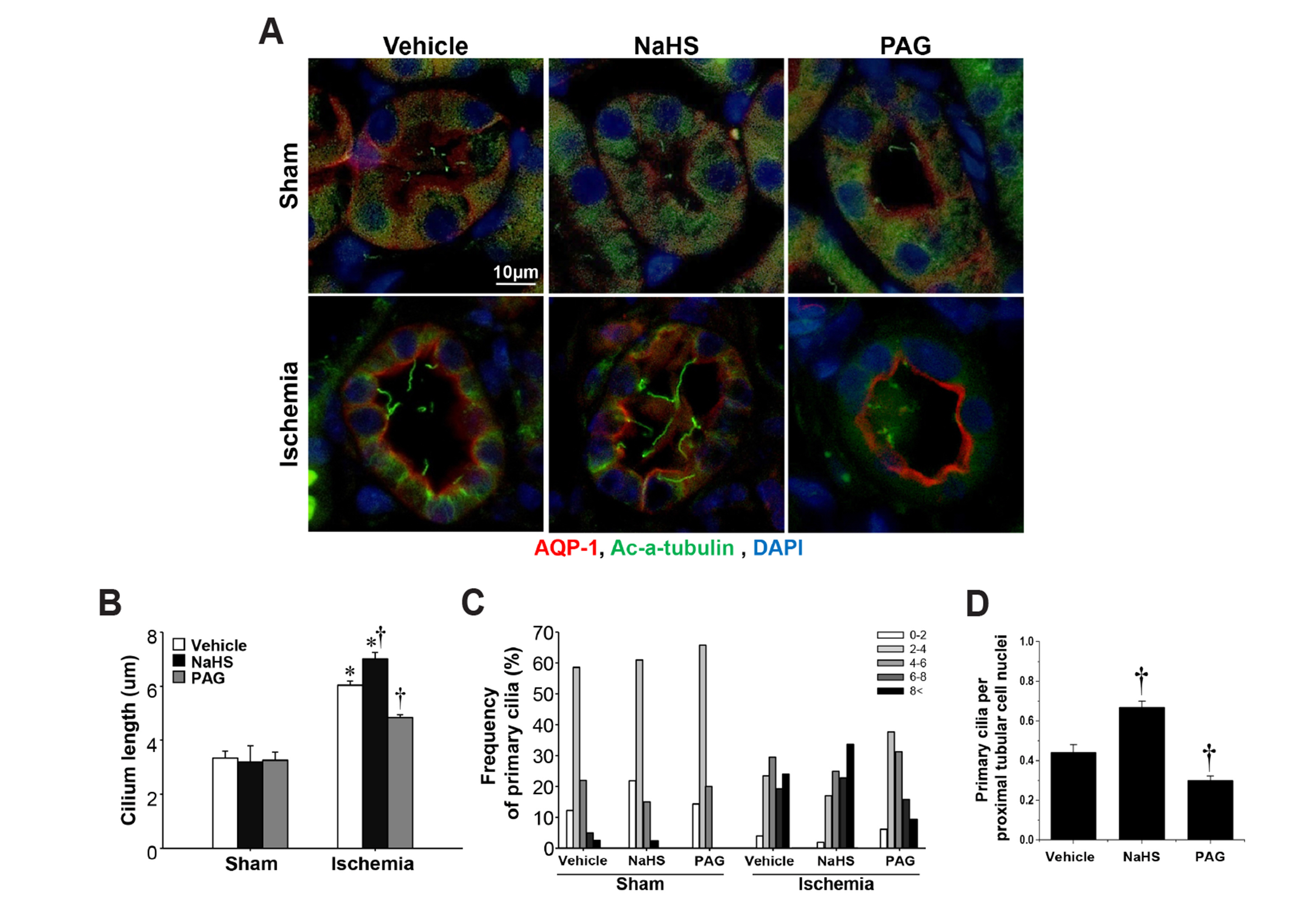
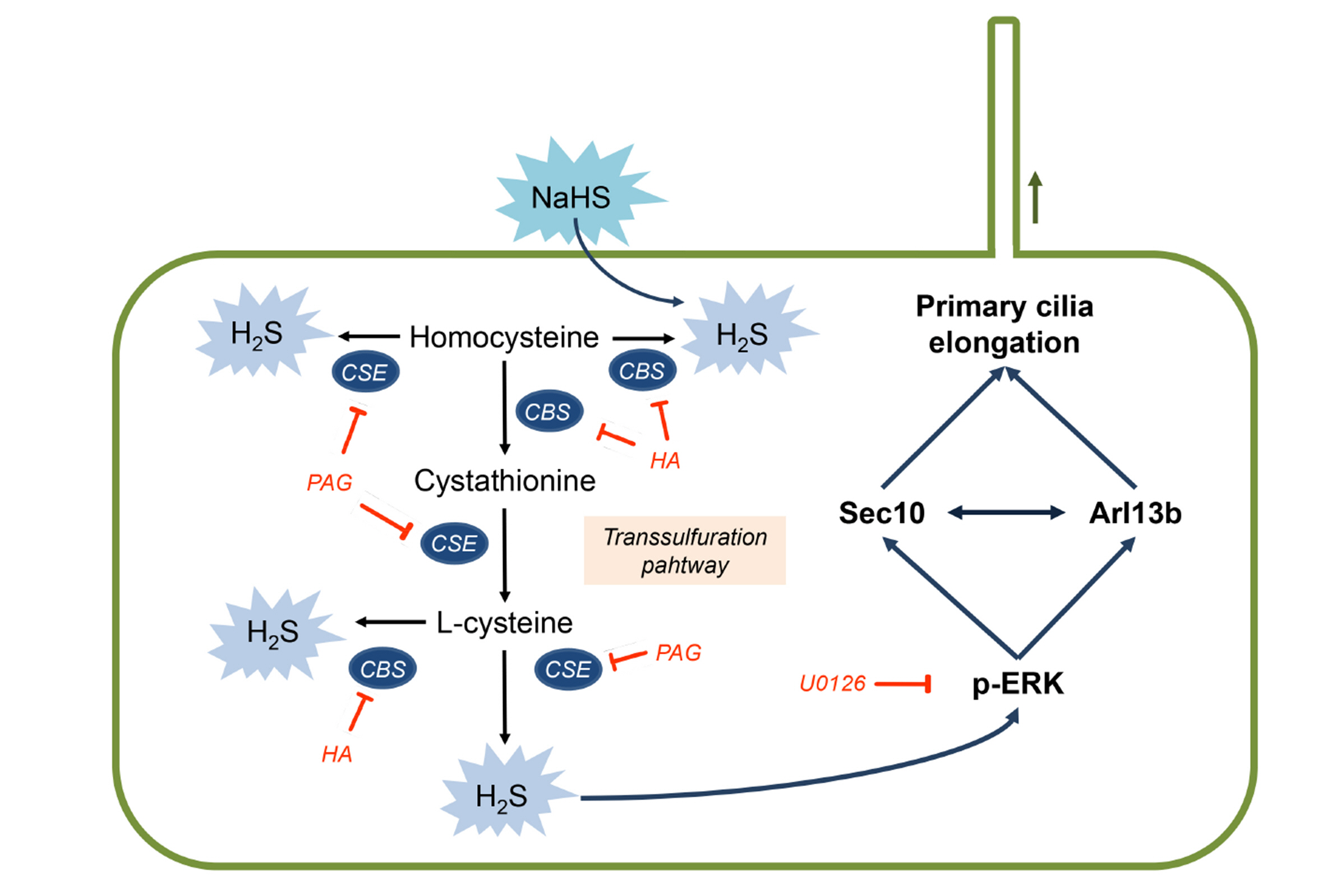
- Primary cilia on kidney tubular cells play crucial roles in maintaining structure and physiological function. Emerging evidence indicates that the absence of primary cilia, and their length, are associated with kidney diseases. The length of primary cilia in kidney tubular epithelial cells depends, at least in part, on oxidative stress and extracellular signal-regulated kinase 1/2 (ERK) activation. Hydrogen sulfide (H2S) is involved in antioxidant systems and the ERK signaling pathway. Therefore, in this study, we investigated the role of H2S in primary cilia elongation and the downstream pathway. In cultured Madin-Darby Canine Kidney cells, the length of primary cilia gradually increased up to 4 days after the cells were grown to confluent monolayers. In addition, the expression of H2S-producing enzyme increased concomitantly with primary cilia length. Treatment with NaHS, an exogenous H2S donor, accelerated the elongation of primary cilia whereas DL-propargylglycine (a cystathionine γ-lyase inhibitor) and hydroxylamine (a cystathionine-β-synthase inhibitor) delayed their elongation. NaHS treatment increased ERK activation and Sec10 and Arl13b protein expression, both of which are involved in cilia formation and elongation. Treatment with U0126, an ERK inhibitor, delayed elongation of primary cilia and blocked the effect of NaHS-mediated primary cilia elongation and Sec10 and Arl13b upregulation. Finally, we also found that H 2 S accelerated primary cilia elongation after ischemic kidney injury. These results indicate that H2S lengthens primary cilia through ERK activation and a consequent increase in Sec10 and Arl13b expression, suggesting that H2S and its downstream targets could be novel molecular targets for regulating primary cilia.
Figure
Reference
-
1. Badano JL, Mitsuma N, Beales PL, Katsanis N. 2006; The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 7:125–148. DOI: 10.1146/annurev.genom.7.080505.115610. PMID: 16722803.
Article2. Bulger RE, Siegel FL, Pendergrass R. 1974; Scanning and transmission electron microscopy of the rat kidney. Am J Anat. 139:483–501. DOI: 10.1002/aja.1001390403. PMID: 4818045.
Article3. Webber WA, Lee J. 1975; Fine structure of mammalian renal cilia. Anat Rec. 182:339–343. DOI: 10.1002/ar.1091820307. PMID: 1155803.
Article4. Praetorius HA, Spring KR. 2003; The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 12:517–520. DOI: 10.1097/00041552-200309000-00006. PMID: 12920399.
Article5. Praetorius HA, Spring KR. 2003; Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 191:69–76. DOI: 10.1007/s00232-002-1042-4. PMID: 12532278.
Article6. Zhang S, Pan C, Zhou F, Yuan Z, Wang H, Cui W, Zhang G. 2015; Hydrogen sulfide as a potential therapeutic target in fibrosis. Oxid Med Cell Longev. 2015:593407. DOI: 10.1155/2015/593407. PMID: 26078809. PMCID: PMC4442300.
Article7. Kim JI, Kim J, Jang HS, Noh MR, Lipschutz JH, Park KM. 2013; Reduction of oxidative stress during recovery accelerates normalization of primary cilia length that is altered after ischemic injury in murine kidneys. Am J Physiol Renal Physiol. 304:F1283–F1294. DOI: 10.1152/ajprenal.00427.2012. PMID: 23515720.
Article8. Wang S, Dong Z. 2013; Primary cilia and kidney injury: current research status and future perspectives. Am J Physiol Renal Physiol. 305:F1085–F1098. DOI: 10.1152/ajprenal.00399.2013. PMID: 23904226. PMCID: PMC3798724.
Article9. Jang HS, Han SJ, Kim JI, Lee S, Lipschutz JH, Park KM. 2013; Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim Biophys Acta. 1832:1998–2008. DOI: 10.1016/j.bbadis.2013.07.001. PMID: 23851027.
Article10. Zuo X, Guo W, Lipschutz JH. 2009; The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 20:2522–2529. DOI: 10.1091/mbc.e08-07-0772. PMID: 19297529. PMCID: PMC2682593.
Article11. Fiorucci S, Distrutti E, Cirino G, Wallace JL. 2006; The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 131:259–271. DOI: 10.1053/j.gastro.2006.02.033. PMID: 16831608.
Article12. Kimura H. 2011; Hydrogen sulfide: its production, release and functions. Amino Acids. 41:113–121. DOI: 10.1007/s00726-010-0510-x. PMID: 20191298.
Article13. Szabó C. 2007; Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 6:917–935. DOI: 10.1038/nrd2425. PMID: 17948022.
Article14. Wang R. 2002; Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 16:1792–1798. DOI: 10.1096/fj.02-0211hyp. PMID: 12409322.15. Banerjee R. 2011; Hydrogen sulfide: redox metabolism and signaling. Antioxid Redox Signal. 15:339–341. DOI: 10.1089/ars.2011.3912. PMID: 21275829. PMCID: PMC3118658.
Article16. Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. 2007; Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 81:1322–1332. DOI: 10.1189/jlb.1006599. PMID: 17289797.17. Cai WJ, Wang MJ, Ju LH, Wang C, Zhu YC. 2010; Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int. 34:565–572. DOI: 10.1042/CBI20090368. PMID: 20184555.
Article18. Han SJ, Kim JI, Park JW, Park KM. 2015; Hydrogen sulfide accelerates the recovery of kidney tubules after renal ischemia/reperfusion injury. Nephrol Dial Transplant. 30:1497–1506. DOI: 10.1093/ndt/gfv226. PMID: 26142397.
Article19. Han SJ, Noh MR, Jung JM, Ishii I, Yoo J, Kim JI, Park KM. 2017; Hydrogen sulfide-producing cystathionine γ-lyase is critical in the progression of kidney fibrosis. Free Radic Biol Med. 112:423–432. DOI: 10.1016/j.freeradbiomed.2017.08.017. PMID: 28842346.
Article20. Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, Papapetropoulos A. 2013; Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol. 169:922–932. DOI: 10.1111/bph.12171. PMID: 23488457. PMCID: PMC3687671.
Article21. Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. 2013; Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta. 1832:1989–1997. DOI: 10.1016/j.bbadis.2013.06.015. PMID: 23846016.
Article22. Fan HN, Wang HJ, Yang-Dan CR, Ren L, Wang C, Li YF, Deng Y. 2013; Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol Med Rep. 7:247–253. DOI: 10.3892/mmr.2012.1153. PMID: 23129058.
Article23. Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. 2007; Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J Appl Physiol (1985). 102:261–268. DOI: 10.1152/japplphysiol.00096.2006. PMID: 17038495.
Article24. Han SJ, Jang HS, Noh MR, Kim J, Kong MJ, Kim JI, Park JW, Park KM. 2017; Mitochondrial NADP+-dependent isocitrate dehydrogenase deficiency exacerbates mitochondrial and cell damage after kidney ischemia-reperfusion injury. J Am Soc Nephrol. 28:1200–1215. DOI: 10.1681/ASN.2016030349. PMID: 27821630. PMCID: PMC5373447.25. Larkins CE, Aviles GD, East MP, Kahn RA, Caspary T. 2011; Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell. 22:4694–4703. DOI: 10.1091/mbc.e10-12-0994. PMID: 21976698. PMCID: PMC3226485.
Article26. Seixas C, Choi SY, Polgar N, Umberger NL, East MP, Zuo X, Moreiras H, Ghossoub R, Benmerah A, Kahn RA, Fogelgren B, Caspary T, Lipschutz JH, Barral DC. 2016; Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell. 27:308–320. DOI: 10.1091/mbc.e15-02-0061. PMID: 26582389. PMCID: PMC4713133.
Article27. Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA. 2009; Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol. 20:2147–2153. DOI: 10.1681/ASN.2008101105. PMID: 19608704. PMCID: PMC2754102.
Article28. Wang L, Weidenfeld R, Verghese E, Ricardo SD, Deane JA. 2008; Alterations in renal cilium length during transient complete ureteral obstruction in the mouse. J Anat. 213:79–85. DOI: 10.1111/j.1469-7580.2008.00918.x. PMID: 18537851. PMCID: PMC2526103.
Article29. Keeling J, Tsiokas L, Maskey D. 2016; Cellular mechanisms of ciliary length control. Cells. 5:6. DOI: 10.3390/cells5010006. PMID: 26840332. PMCID: PMC4810091.
Article30. Avasthi P, Marshall WF. 2012; Stages of ciliogenesis and regulation of ciliary length. Differentiation. 83:S30–S42. DOI: 10.1016/j.diff.2011.11.015. PMID: 22178116. PMCID: PMC3269565.
Article31. Han SJ, Jung JK, Im SS, Lee SR, Jang BC, Park KM, Kim JI. 2018; Deficiency of primary cilia in kidney epithelial cells induces epithelial to mesenchymal transition. Biochem Biophys Res Commun. 496:450–454. DOI: 10.1016/j.bbrc.2018.01.079. PMID: 29337054.
Article32. Han SJ, Jang HS, Kim JI, Lipschutz JH, Park KM. 2016; Unilateral nephrectomy elongates primary cilia in the remaining kidney via reactive oxygen species. Sci Rep. 6:22281. DOI: 10.1038/srep22281. PMID: 26923764. PMCID: PMC4770282.
Article33. Lipschutz JH, Guo W, O'Brien LE, Nguyen YH, Novick P, Mostov KE. 2000; Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol Biol Cell. 11:4259–4275. DOI: 10.1091/mbc.11.12.4259. PMID: 11102522. PMCID: PMC15071.
Article34. Park KM, Fogelgren B, Zuo X, Kim J, Chung DC, Lipschutz JH. 2010; Exocyst Sec10 protects epithelial barrier integrity and enhances recovery following oxidative stress, by activation of the MAPK pathway. Am J Physiol Renal Physiol. 298:F818–F826. DOI: 10.1152/ajprenal.00596.2009. PMID: 20053792. PMCID: PMC2838587.
Article35. Yang G, Sun X, Wang R. 2004; Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 18:1782–1784. DOI: 10.1096/fj.04-2279fje. PMID: 15371330.
Article36. Liu D, Wang Z, Zhan J, Zhang Q, Wang J, Zhang Q, Xian X, Luan Q, Hao A. 2014; Hydrogen sulfide promotes proliferation and neuronal differentiation of neural stem cells and protects hypoxia-induced decrease in hippocampal neurogenesis. Pharmacol Biochem Behav. 116:55–63. DOI: 10.1016/j.pbb.2013.11.009. PMID: 24246910.
Article37. Choi DE, Jeong JY, Choi H, Chang YK, Ahn MS, Ham YR, Na KR, Lee KW. 2017; ERK phosphorylation plays an important role in the protection afforded by hypothermia against renal ischemia-reperfusion injury. Surgery. 161:444–452. DOI: 10.1016/j.surg.2016.07.028. PMID: 27590616.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can Tissue Cilia Lengths and Urine Cilia Proteins Be Markers of Kidney Diseases?
- Beneficial Role of Hydrogen Sulfide in Renal Ischemia Reperfusion Injury in Rats
- Cyclosporin A aggravates hydrogen peroxide-induced cell death in kidney proximal tubule epithelial cells
- Pathogenesis and New Treatment of Autosomal Dominant Polycystic Kidney Disease
- Hydrogen sulfide ameliorates abdominal aorta coarctationinduced myocardial fibrosis by inhibiting pyroptosis through regulating eukaryotic translation initiation factor 2αα phosphorylation and activating PI3K/AKT1 pathway