Chonnam Med J.
2018 May;54(2):83-89. 10.4068/cmj.2018.54.2.83.
Can Tissue Cilia Lengths and Urine Cilia Proteins Be Markers of Kidney Diseases?
- Affiliations
-
- 1Department of Anatomy and BK21 Plus, School of Medicine, Kyungpook National University, Daegu, Korea. kmpark@knu.ac.kr
- KMID: 2411891
- DOI: http://doi.org/10.4068/cmj.2018.54.2.83
Abstract
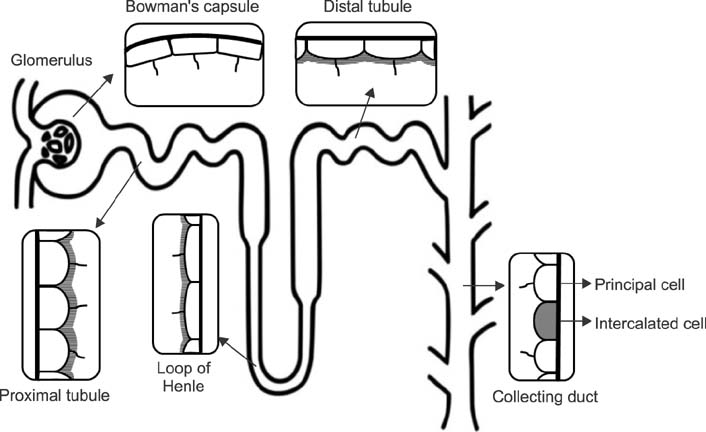
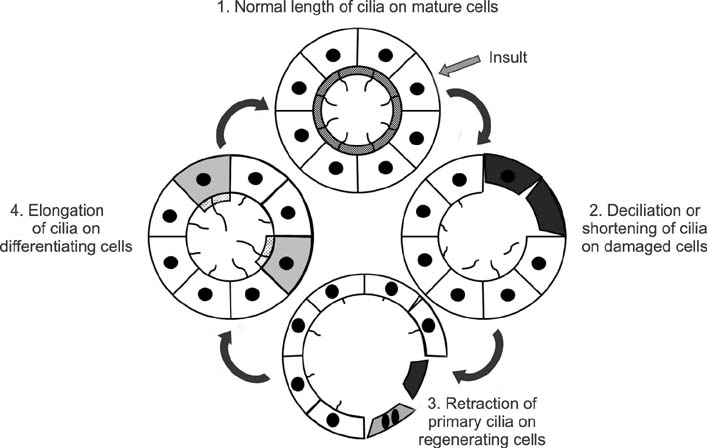
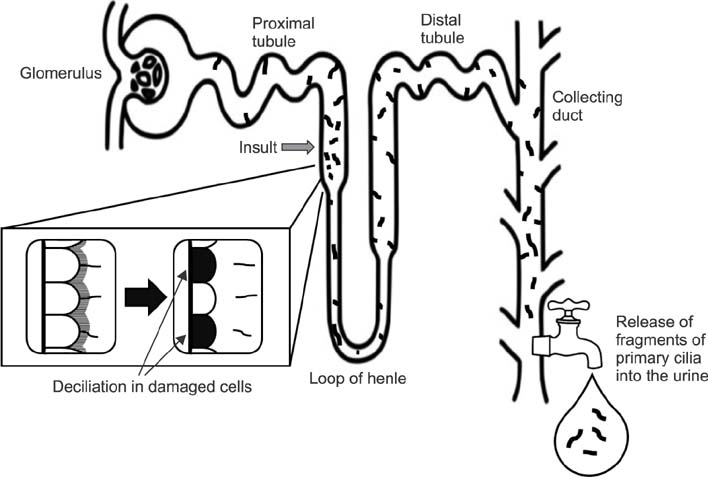
- The primary cilium is an organelle which consists of a microtubule in the core and a surrounding cilia membrane, and has long been recognized as a "vestigial organelle". However, new evidence demonstrates that the primary cilium has a notable effect on signal transduction in the cell and is associated with some genetic and non-genetic diseases. In the kidney, the primary cilium protrudes into the Bowman's space and the tubular lumen from the apical side of epithelial cells. The length of primary cilia is dynamically altered during the normal cell cycle, being shortened by retraction into the cell body at the entry of cell division and elongated at differentiation. Furthermore, the length of primary cilia is also dynamically changed in the cells, as a result and/or cause, during the progression of various kidney diseases including acute kidney injury and chronic kidney disease. Notably, recent data has demonstrated that the shortening of the primary cilium in the cell is associated with fragmentation, apart from retraction into the cell body, in the progression of diseases and that the fragmented primary cilia are released into the urine. This data reveals that the alteration of primary cilia length could be related to the progression of diseases. This review will consider if primary cilia length alteration is associated with the progression of kidney diseases and if the length of tissue primary cilia and the presence or increase of cilia proteins in the urine is indicative of kidney diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007; 69:377–400.
Article2. Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010; 123:499–503.
Article3. Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005; 67:515–529.
Article4. Boletta A, Germino GG. Role of polycystins in renal tubulogenesis. Trends Cell Biol. 2003; 13:484–492.
Article5. Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol. 2009; 20:2147–2153.
Article6. Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA. Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant. 2008; 23:834–841.
Article7. Han SJ, Jang HS, Seu SY, Cho HJ, Hwang YJ, Kim JI, et al. Hepatic ischemia/reperfusion injury disrupts the homeostasis of kidney primary cilia via oxidative stress. Biochim Biophys Acta. 2017; 1863:1817–1828.
Article8. Han SJ, Jang HS, Kim JI, Lipschutz JH, Park KM. Unilateral nephrectomy elongates primary cilia in the remaining kidney via reactive oxygen species. Sci Rep. 2016; 6:22281.
Article9. Kim JI, Kim J, Jang HS, Noh MR, Lipschutz JH, Park KM. Reduction of oxidative stress during recovery accelerates normalization of primary cilia length that is altered after ischemic injury in murine kidneys. Am J Physiol Renal Physiol. 2013; 304:F1283–F1294.
Article10. Bernabé-Rubio M, Alonso MA. Routes and machinery of primary cilium biogenesis. Cell Mol Life Sci. 2017; 74:4077–4095.
Article11. Wang L, Weidenfeld R, Verghese E, Ricardo SD, Deane JA. Alterations in renal cilium length during transient complete ureteral obstruction in the mouse. J Anat. 2008; 213:79–85.
Article12. Song Y, Brady ST. Post-translational modifications of tubulin: pathways to functional diversity of microtubules. Trends Cell Biol. 2015; 25:125–136.
Article13. Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005; 169:707–710.
Article14. Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008; 17:1578–1590.
Article15. Seeley ES, Nachury MV. The perennial organelle: assembly and disassembly of the primary cilium. J Cell Sci. 2010; 123:511–518.
Article16. Miyoshi K, Kasahara K, Miyazaki I, Asanuma M. Factors that influence primary cilium length. Acta Med Okayama. 2011; 65:279–285.17. Lee JE, Gleeson JG. A systems-biology approach to understanding the ciliopathy disorders. Genome Med. 2011; 3:59.
Article18. Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003; 12:517–520.
Article19. Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, et al. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010; 464:1048–1051.
Article20. Overgaard CE, Sanzone KM, Spiczka KS, Sheff DR, Sandra A, Yeaman C. Deciliation is associated with dramatic remodeling of epithelial cell junctions and surface domains. Mol Biol Cell. 2009; 20:102–113.
Article21. Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010; 25:279–292.
Article22. Schneider L, Stock CM, Dieterich P, Jensen BH, Pedersen LB, Satir P, et al. The Na+/H+ exchanger NHE1 is required for directional migration stimulated via PDGFR-alpha in the primary cilium. J Cell Biol. 2009; 185:163–176.
Article23. Rodríguez-Ribera L, Slattery C, Mc Morrow T, Marcos R, Pastor S. Reactive carbonyl compounds impair wound healing by vimentin collapse and loss of the primary cilium. Food Chem Toxicol. 2017; 108:128–138.
Article24. Ichii O, Kimura J, Okamura T, Horino T, Nakamura T, Sasaki H, et al. IL-36α Regulates Tubulointerstitial Inflammation in the Mouse Kidney. Front Immunol. 2017; 8:1346.
Article25. Verghese E, Zhuang J, Saiti D, Ricardo SD, Deane JA. In vitro investigation of renal epithelial injury suggests that primary cilium length is regulated by hypoxia-inducible mechanisms. Cell Biol Int. 2011; 35:909–913.26. Wann AK, Knight MM. Primary cilia elongation in response to interleukin-1 mediates the inflammatory response. Cell Mol Life Sci. 2012; 69:2967–2977.
Article27. Jang HS, Han SJ, Kim JI, Lee S, Lipschutz JH, Park KM. Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/ reperfusion injury. Biochim Biophys Acta. 2013; 1832:1998–2008.
Article28. Song J, Rolfe BE, Campbell JH, Campbell GR. Changes in three-dimensional architecture of microfilaments in cultured vascular smooth muscle cells during phenotypic modulation. Tissue Cell. 1998; 30:324–333.
Article29. Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003; 15:105–110.
Article30. Deane JA, Ricardo SD. Emerging roles for renal primary cilia in epithelial repair. Int Rev Cell Mol Biol. 2012; 293:169–193.
Article31. Bulger RE, Siegel FL, Pendergrass R. Scanning and transmission electron microscopy of the rat kidney. Am J Anat. 1974; 139:483–501.
Article32. Webber WA, Lee J. Fine structure of mammalian renal cilia. Anat Rec. 1975; 182:339–343.
Article33. Andrews PM, Porter KR. A scanning electron microscopic study of the nephron. Am J Anat. 1974; 140:81–115.
Article34. Saraga-Babić M, Vukojević K, Bočina I, Drnašin K, Saraga M. Ciliogenesis in normal human kidney development and post-natal life. Pediatr Nephrol. 2012; 27:55–63.
Article35. Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000; 151:709–718.
Article36. Sung CH, Li A. Ciliary resorption modulates G1 length and cell cycle progression. Cell Cycle. 2011; 10:2825–2826.
Article37. Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002; 3:813–825.
Article38. Bossinger O, Bachmann A. Ciliogenesis: polarity proteins on the move. Curr Biol. 2004; 14:R844–R846.
Article39. Nag S, Resnick A. Biophysics and biofluid dynamics of primary cilia: evidence for and against the flow-sensing function. Am J Physiol Renal Physiol. 2017; 313:F706–F720.
Article40. Cole DG, Snell WJ. SnapShot: Intraflagellar transport. Cell. 2009; 137:784–784.
Article41. Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008; 85:23–61.42. Wang S, Dong Z. Primary cilia and kidney injury: current research status and future perspectives. Am J Physiol Renal Physiol. 2013; 305:F1085–F1098.
Article43. Han SJ, Kim JH, Kim JI, Park KM. Inhibition of microtubule dynamics impedes repair of kidney ischemia/reperfusion injury and increases fibrosis. Sci Rep. 2016; 6:27775.
Article44. Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell. 2011; 22:806–816.
Article45. Kim S, Tsiokas L. Cilia and cell cycle re-entry: more than a coincidence. Cell Cycle. 2011; 10:2683–2690.46. Goggolidou P, Wilson PD. Novel biomarkers in kidney disease: roles for cilia, Wnt signalling and ATMIN in polycystic kidney disease. Biochem Soc Trans. 2016; 44:1745–1751.
Article47. Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009; 297:F461–F470.
Article48. Kim J, Jung KJ, Park KM. Reactive oxygen species differently regulate renal tubular epithelial and interstitial cell proliferation after ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2010; 298:F1118–F1129.
Article49. Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med (Berl). 2007; 85:1325–1330.
Article50. Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003; 100:5286–5291.
Article51. Basten SG, Giles RH. Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia. 2013; 2:6.
Article52. Basten SG, Willekers S, Vermaat JS, Slaats GG, Voest EE, van Diest PJ, et al. Reduced cilia frequencies in human renal cell carcinomas versus neighboring parenchymal tissue. Cilia. 2013; 2:2.
Article53. Saito S, Tampe B, Müller GA, Zeisberg M. Primary cilia modulate balance of canonical and non-canonical Wnt signaling responses in the injured kidney. Fibrogenesis Tissue Repair. 2015; 8:6.
Article54. Halt K, Vainio S. Coordination of kidney organogenesis by Wnt signaling. Pediatr Nephrol. 2014; 29:737–744.
Article55. Oh EC, Katsanis N. Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol. 2013; 24:10–18.
Article56. Rodat-Despoix L, Delmas P. Ciliar functions in the nephron. Pflugers Arch. 2009; 458:179–187.
Article57. Abdul-Majeed S, Moloney BC, Nauli SM. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci. 2012; 69:165–173.
Article58. Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007; 129:1351–1363.
Article59. Xu Z, Schaedel L, Portran D, Aguilar A, Gaillard J, Marinkovich MP, et al. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science. 2017; 356:328–332.
Article60. Wang S, Livingston MJ, Su Y, Dong Z. Reciprocal regulation of cilia and autophagy via the MTOR and proteasome pathways. Autophagy. 2015; 11:607–616.
Article61. Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001; 276:11870–11876.
Article62. Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem. 2004; 279:52282–52292.
Article63. Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993; 43:1160–1178.
Article64. Bonventre JV. Kidney injury molecule-1: a translational journey. Trans Am Clin Climatol Assoc. 2014; 125:293–299.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathogenesis and New Treatment of Autosomal Dominant Polycystic Kidney Disease
- Interplay Between Primary Cilia and Autophagy and Its Controversial Roles in Cancer
- Shortening of primary cilia length is associated with urine concentration in the kidneys
- Cyst growth, polycystins, and primary cilia in autosomal dominant polycystic kidney disease
- Rediscovering Primary Cilia in Pancreatic Islets