Cancer Res Treat.
2021 Jul;53(3):685-694. 10.4143/crt.2020.1015.
Synergistic Tumoricidal Effects of Alpha-Lipoic Acid and Radiotherapy on Human Breast Cancer Cells via HMGB1
- Affiliations
-
- 1Department of Radiation Oncology, Gyeongsang National University Changwon Hospital, Gyeongsang National University College of Medicine, Changwon, Korea
- 2Institute of Health Science, Gyeongsang National University, Jinju, Korea
- 3Biomedical Research Institute, Gyeongsang National University Hospital, Jinju, Korea
- 4Department of Radiation Oncology, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea
- KMID: 2518392
- DOI: http://doi.org/10.4143/crt.2020.1015
Abstract
- Purpose
Radiotherapy (RT) is one of main strategies of cancer treatment. However, some cancer cells are resistant to radiation-induced cell death, including apoptosis. Therefore, alternative approaches targeting different anti-tumor mechanisms such as cell senescence are required. This study aimed to investigate the synergistic effect of alpha-lipoic acid (ALA) on radiation-induced cell death and senescence in MDA-MB-231 human breast cancer cells.
Materials and Methods
The cells were divided into four groups depending on the cell treatment (control, ALA, RT, and ALA+RT). Cells were analyzed for morphology, apoptotic cell death, mitochondrial reactive oxygen species, membrane potential, cellular senescence, and cell cycle.
Results
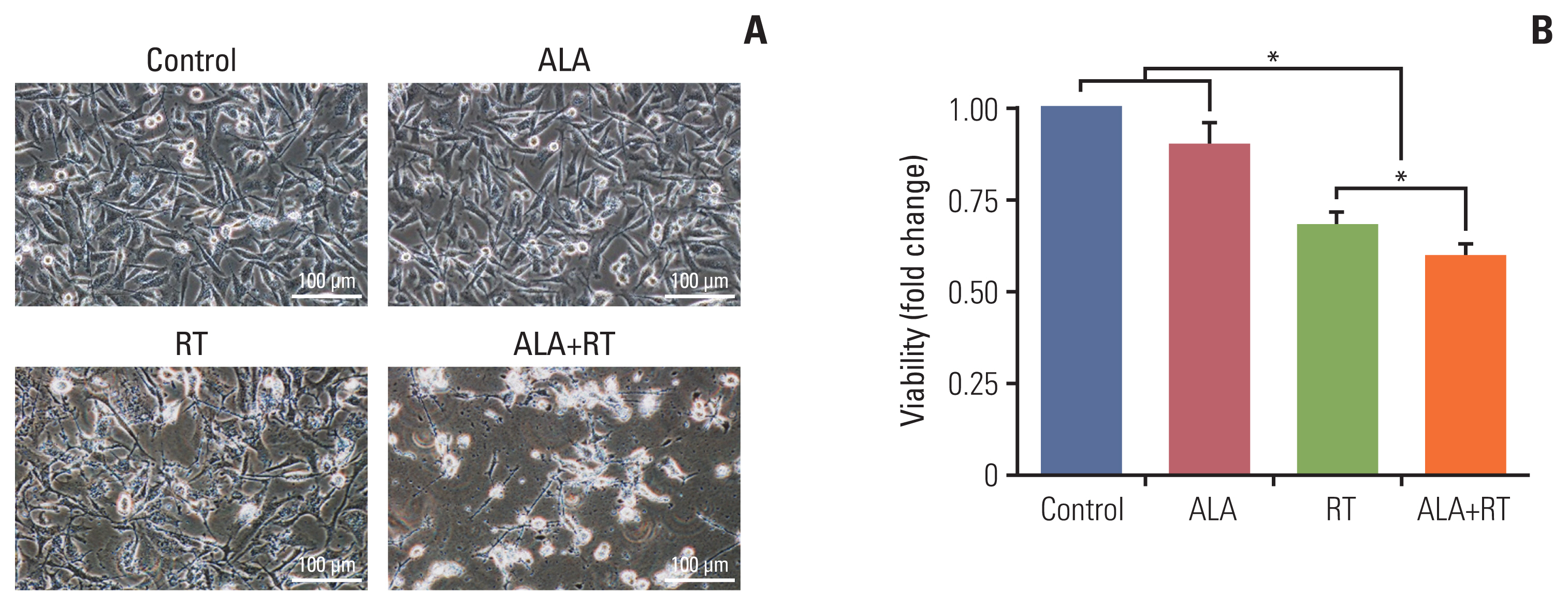
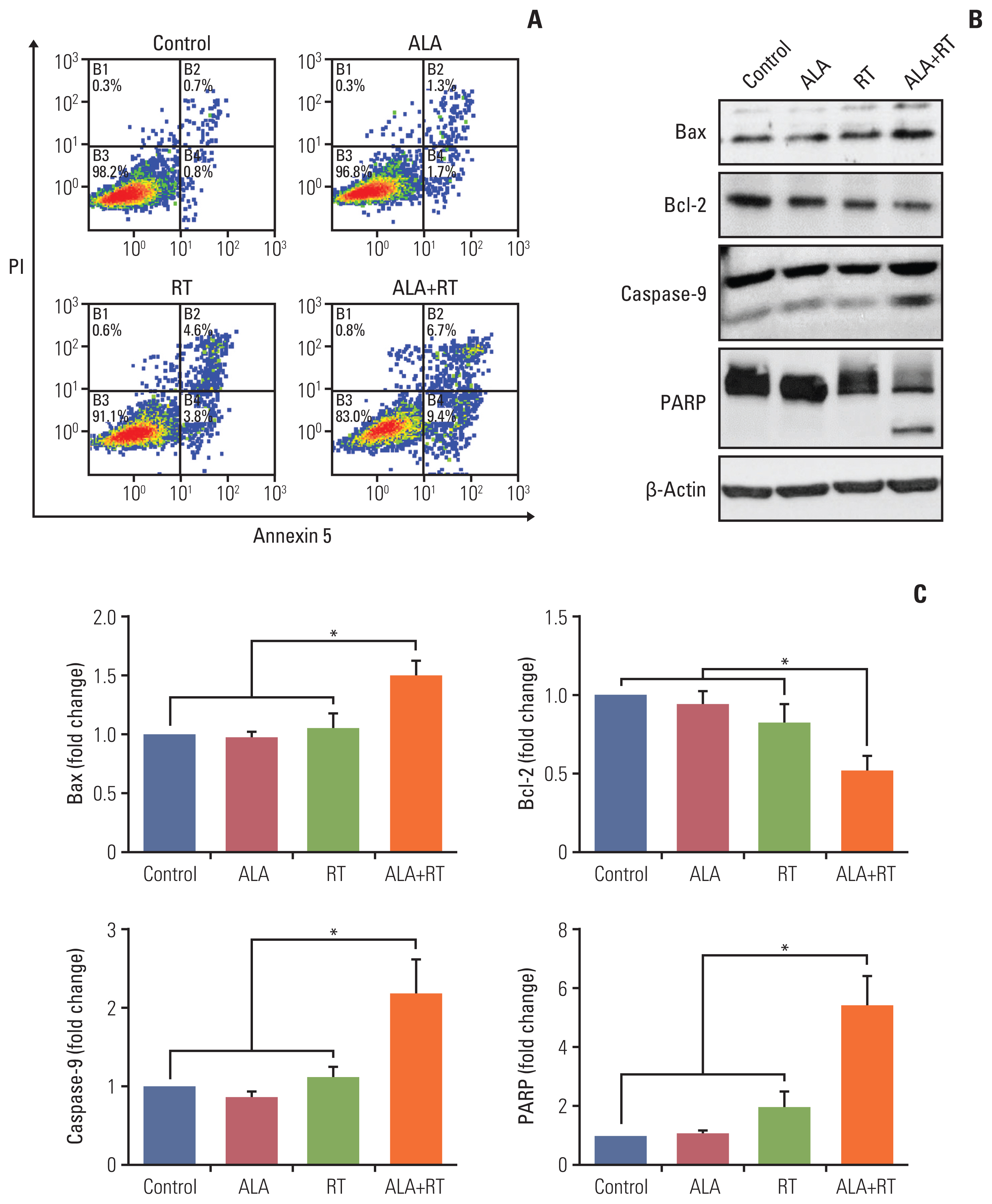
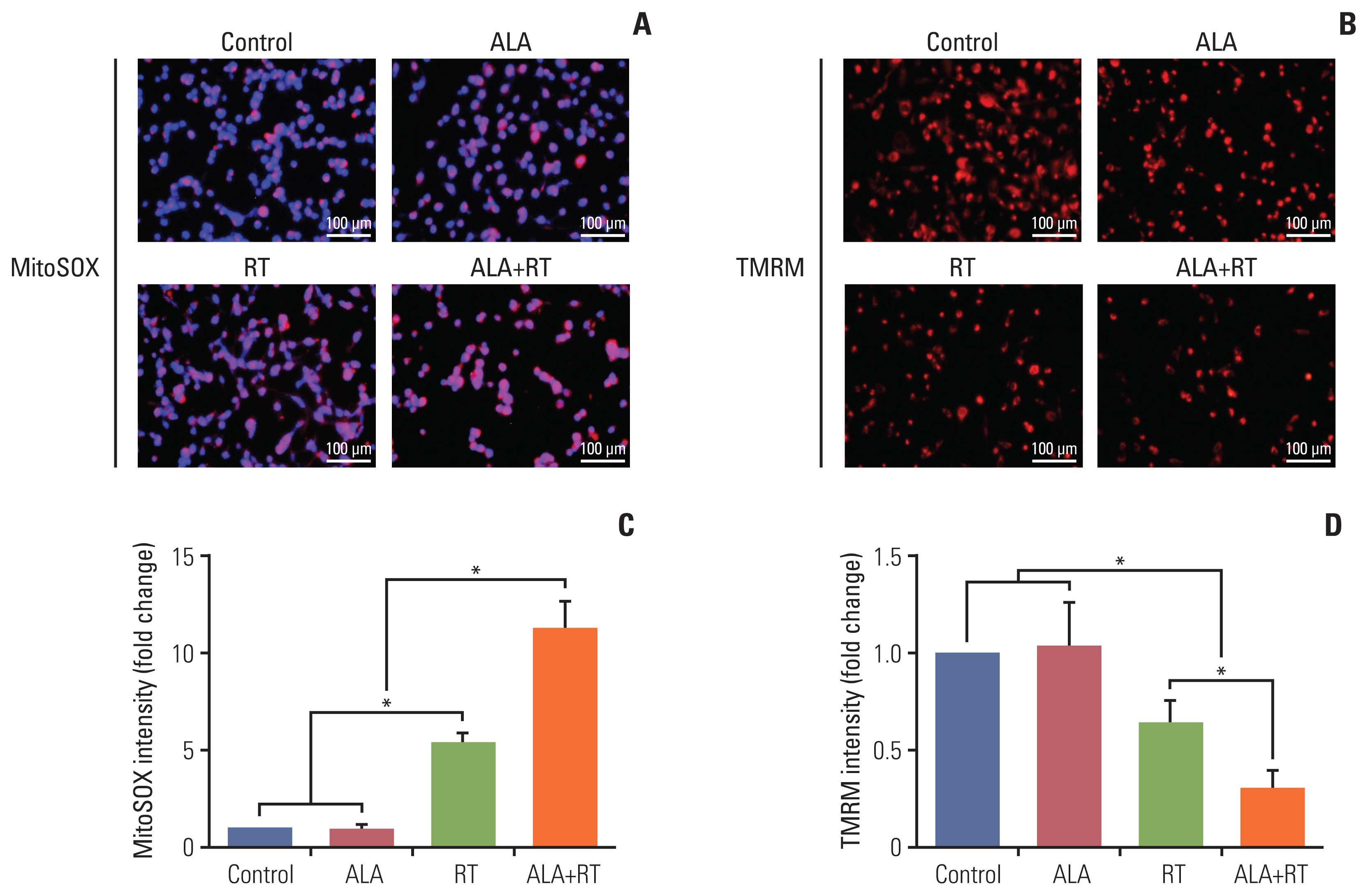
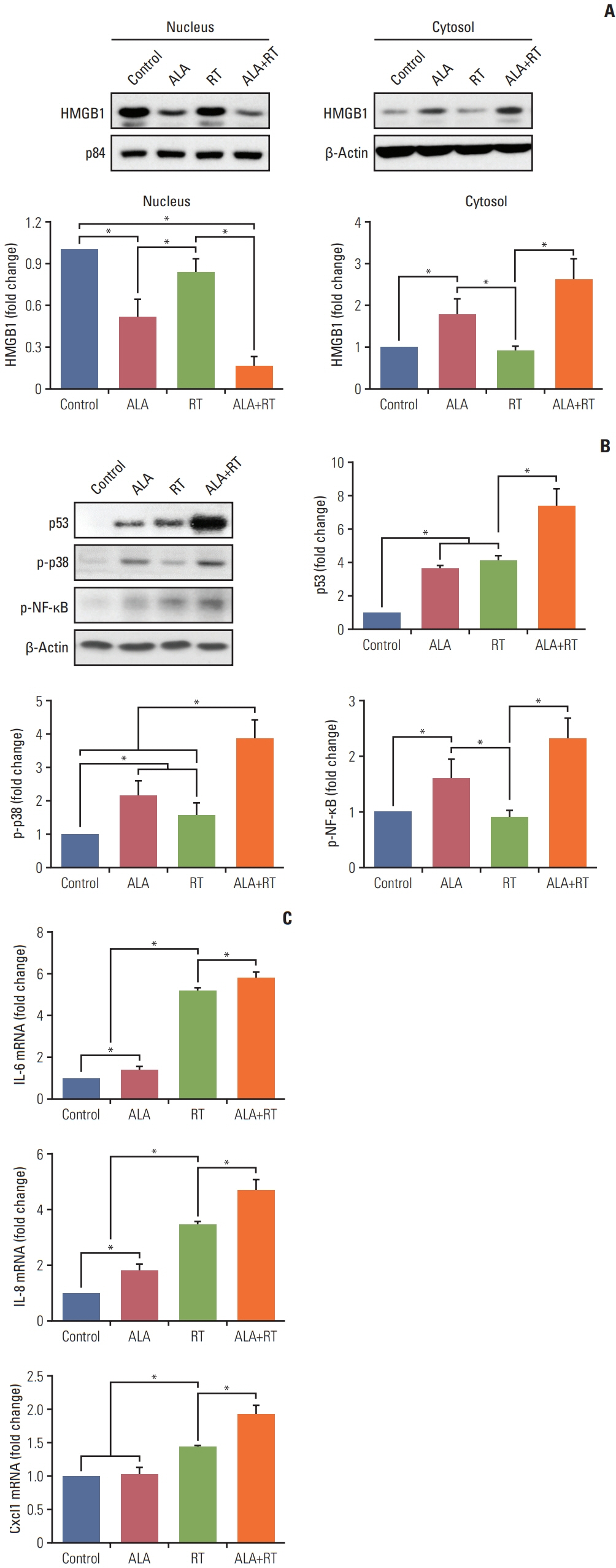
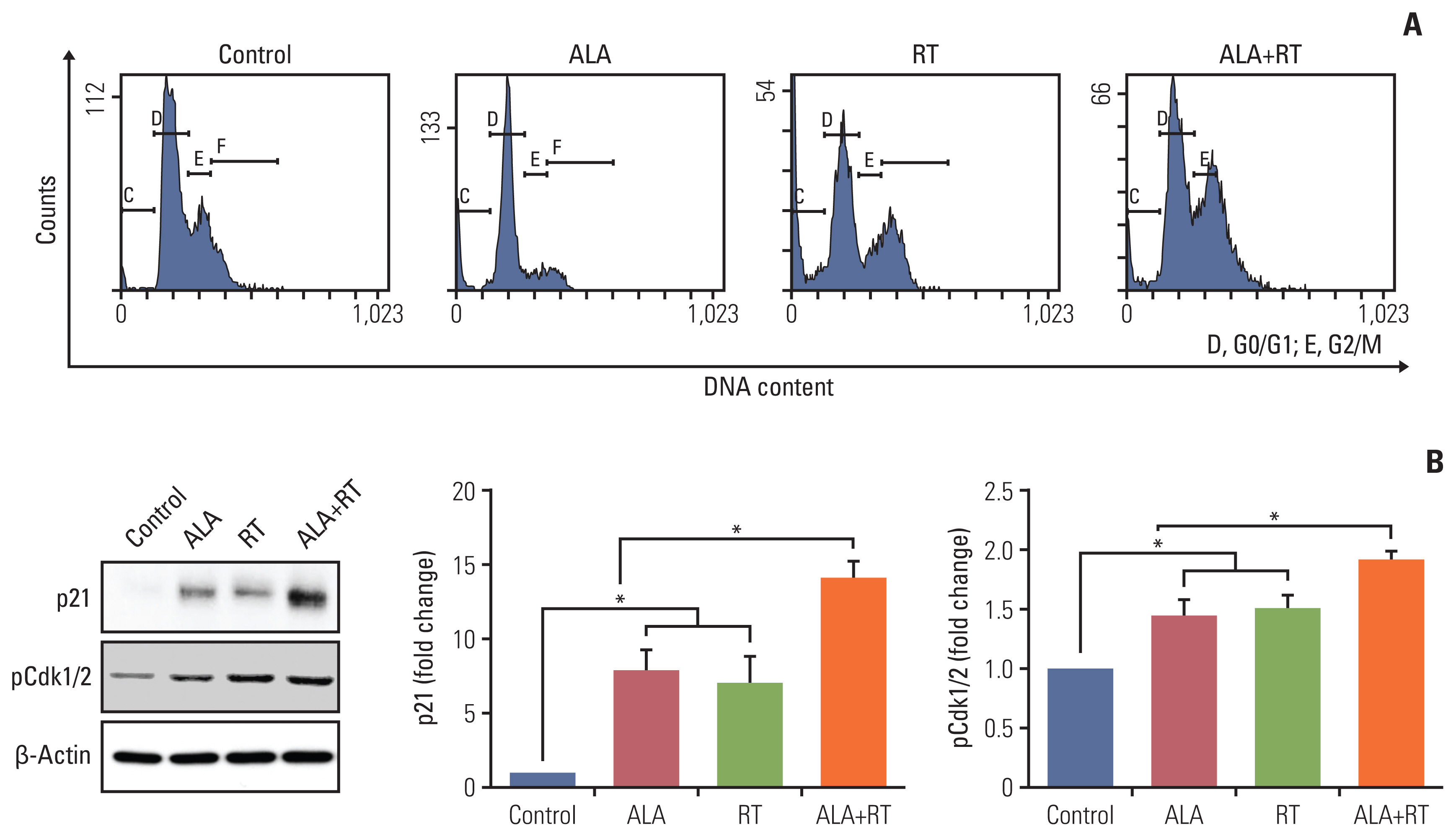
Our data showed that ALA significantly promoted apoptotic cell death when combined with RT, as reflected by Annexin V staining, expression of apoptosis-related factors, mitochondrial damages as well as cell morphological changes and reduction of cell numbers. In addition, ALA significantly enhanced radiation-induced cellular senescence, which was shown by increased HMGB1 expression in the cytosol fraction compared to the control, increased p53 expression compared to the control, activation of p38 as well as nuclear factor кB, and G2/M cell cycle arrest.
Conclusion
The current study is the first report showing a new mode of action (senescence induction) of ALA beyond apoptotic cell death in MDA-MB-231 cancer cells known to be resistant to RT.
Keyword
Figure
Reference
-
References
1. Eom KY, Cho BJ, Choi EJ, Kim JH, Chie EK, Wu HG, et al. The effect of chemoradiotherapy with SRC tyrosine kinase inhibitor, PP2 and temozolomide on malignant glioma cells in vitro and in vivo. Cancer Res Treat. 2016; 48:687–97.2. Kim JH, Moon SH, No M, Kim JJ, Choi EJ, Cho BJ, et al. Isotype-specific inhibition of histone deacetylases: identification of optimal targets for radiosensitization. Cancer Res Treat. 2016; 48:1130–40.
Article3. Raviraj J, Bokkasam VK, Kumar VS, Reddy US, Suman V. Radiosensitizers, radioprotectors, and radiation mitigators. Indian J Dent Res. 2014; 25:83–90.
Article4. Salehi B, Berkay Yilmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, et al. Insights on the use of alpha-lipoic acid for therapeutic purposes. Biomolecules. 2019; 9:356.5. Feuerecker B, Pirsig S, Seidl C, Aichler M, Feuchtinger A, Bruchelt G, et al. Lipoic acid inhibits cell proliferation of tumor cells in vitro and in vivo. Cancer Biol Ther. 2012; 13:1425–35.
Article6. Dorsam B, Goder A, Seiwert N, Kaina B, Fahrer J. Lipoic acid induces p53-independent cell death in colorectal cancer cells and potentiates the cytotoxicity of 5-fluorouracil. Arch Toxicol. 2015; 89:1829–46.
Article7. Puchsaka P, Chaotham C, Chanvorachote P. alpha-Lipoic acid sensitizes lung cancer cells to chemotherapeutic agents and anoikis via integrin beta1/beta3 downregulation. Int J Oncol. 2016; 49:1445–56.8. Tripathy J, Chowdhury AR, Prusty M, Muduli K, Priyadarshini N, Reddy KS, et al. alpha-Lipoic acid prevents the ionizing radiation-induced epithelial-mesenchymal transition and enhances the radiosensitivity in breast cancer cells. Eur J Pharmacol. 2020; 871:172938.9. Kim JH, Jung MH, Kim JP, Kim HJ, Jung JH, Hahm JR, et al. Alpha lipoic acid attenuates radiation-induced oral mucositis in rats. Oncotarget. 2017; 8:72739–47.
Article10. Jeong BK, Song JH, Jeong H, Choi HS, Jung JH, Hahm JR, et al. Effect of alpha-lipoic acid on radiation-induced small intestine injury in mice. Oncotarget. 2016; 7:15105–17.
Article11. Gray M, Turnbull AK, Ward C, Meehan J, Martinez-Perez C, Bonello M, et al. Development and characterisation of acquired radioresistant breast cancer cell lines. Radiat Oncol. 2019; 14:64.
Article12. Qin S, Schulte BA, Wang GY. Role of senescence induction in cancer treatment. World J Clin Oncol. 2018; 9:180–7.
Article13. Ewald JA, Desotelle JA, Wilding G, Jarrard DF. Therapy-induced senescence in cancer. J Natl Cancer Inst. 2010; 102:1536–46.
Article14. Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011; 30:1536–48.
Article15. Lee JJ, Park IH, Rhee WJ, Kim HS, Shin JS. HMGB1 modulates the balance between senescence and apoptosis in response to genotoxic stress. FASEB J. 2019; 33:10942–53.
Article16. Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, et al. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol. 2013; 201:613–29.
Article17. Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010; 5:99–118.18. Mao Z, Ke Z, Gorbunova V, Seluanov A. Replicatively senescent cells are arrested in G1 and G2 phases. Aging (Albany NY). 2012; 4:431–5.
Article19. Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys. 1995; 33:781–96.
Article20. Suzuki M, Boothman DA. Stress-induced premature senescence (SIPS): influence of SIPS on radiotherapy. J Radiat Res. 2008; 49:105–12.21. Chen WS, Yu YC, Lee YJ, Chen JH, Hsu HY, Chiu SJ. Depletion of securin induces senescence after irradiation and enhances radiosensitivity in human cancer cells regardless of functional p53 expression. Int J Radiat Oncol Biol Phys. 2010; 77:566–74.
Article22. Banerjee S, Kundu TK. The acidic C-terminal domain and A-box of HMGB-1 regulates p53-mediated transcription. Nucleic Acids Res. 2003; 31:3236–47.
Article23. Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011; 14:1315–35.
Article24. Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018; 64:127–34.
Article25. Kam WW, Banati RB. Effects of ionizing radiation on mitochondria. Free Radic Biol Med. 2013; 65:607–19.
Article26. Richardson RB, Harper ME. Mitochondrial stress controls the radiosensitivity of the oxygen effect: implications for radiotherapy. Oncotarget. 2016; 7:21469–83.
Article27. Kalinovic S, Oelze M, Kroller-Schon S, Steven S, Vujacic-Mirski K, Kvandova M, et al. Comparison of mitochondrial superoxide detection ex vivo/in vivo by mitoSOX HPLC method with classical assays in three different animal models of oxidative stress. Antioxidants (Basel). 2019; 8:514.
Article28. Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013; 8:2003–14.29. Yang Y, Fang E, Luo J, Wu H, Jiang Y, Liu Y, et al. The antioxidant alpha-lipoic acid inhibits proliferation and invasion of human gastric cancer cells via suppression of STAT3-mediated MUC4 gene expression. Oxid Med Cell Longev. 2019; 2019:3643715.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells
- Synergistic Cytotoxic Effects of Zole-dronic Acid and Radiation in the MCF-7 Human Breast Cancer Cell Line
- Letter: Effects of alpha-lipoic Acid on Differentiation of Thyroid Cancer Cells
- Response: Effects of alpha-lipoic Acid on Differentiation of Thyroid Cancer Cells
- Efficacy and Safety of α-Lipoic Acid and Low Dose Pregabalin Combination in Painful Diabetic Neuropathy