Korean J Physiol Pharmacol.
2021 Jul;25(4):281-296. 10.4196/kjpp.2021.25.4.281.
Whole body hypoxic preconditioning-mediated multiorgan protection in db/db mice via nitric oxide-BDNF-GSK-3β-Nrf2 signaling pathway
- Affiliations
-
- 1Cadre Ward the No.901 Hospital of the Joint Logistics Support Unit of the Chinese People's Liberation Army, Hefei, Anhui 230031, P.R. China
- KMID: 2516868
- DOI: http://doi.org/10.4196/kjpp.2021.25.4.281
Abstract
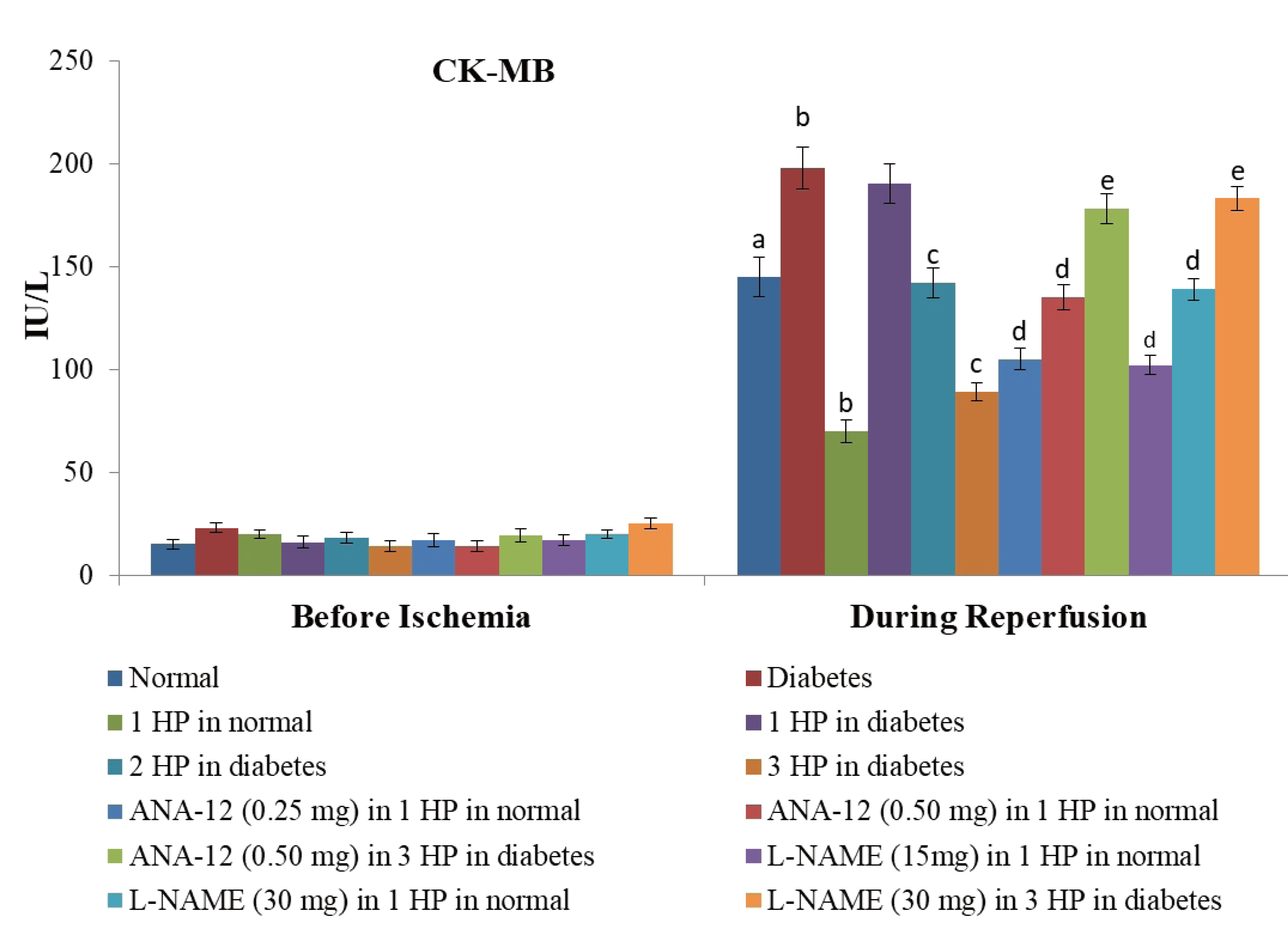
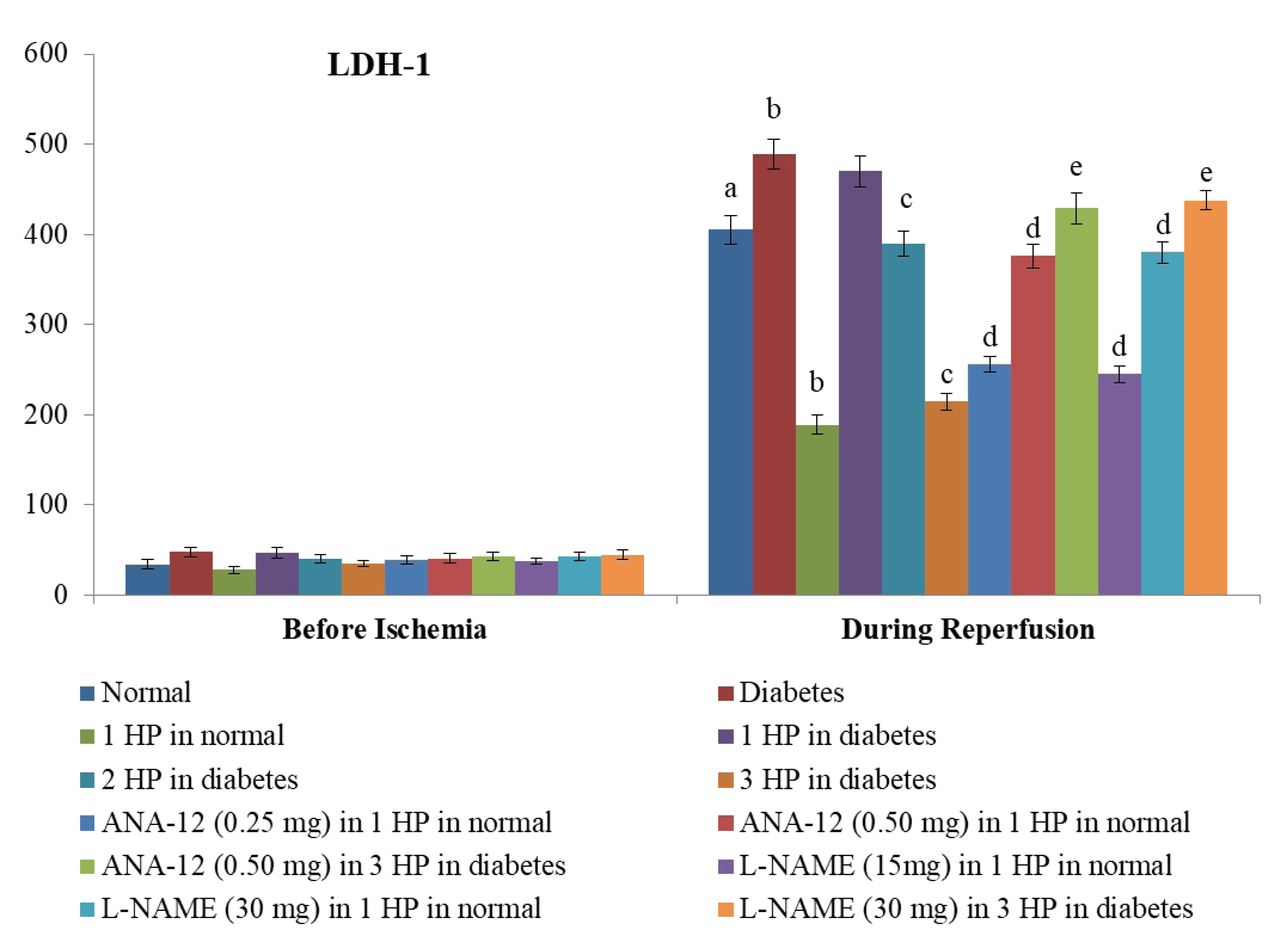
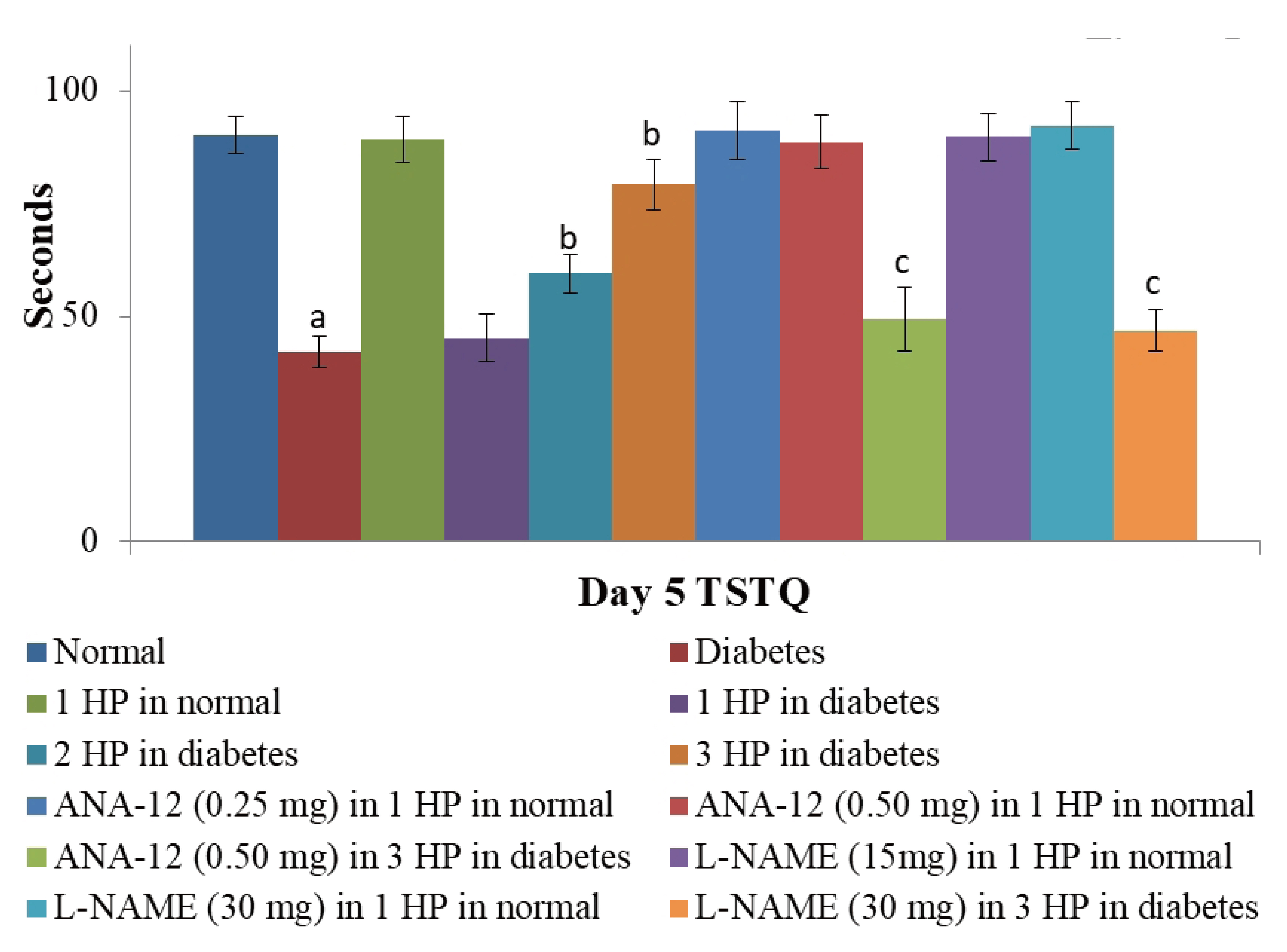
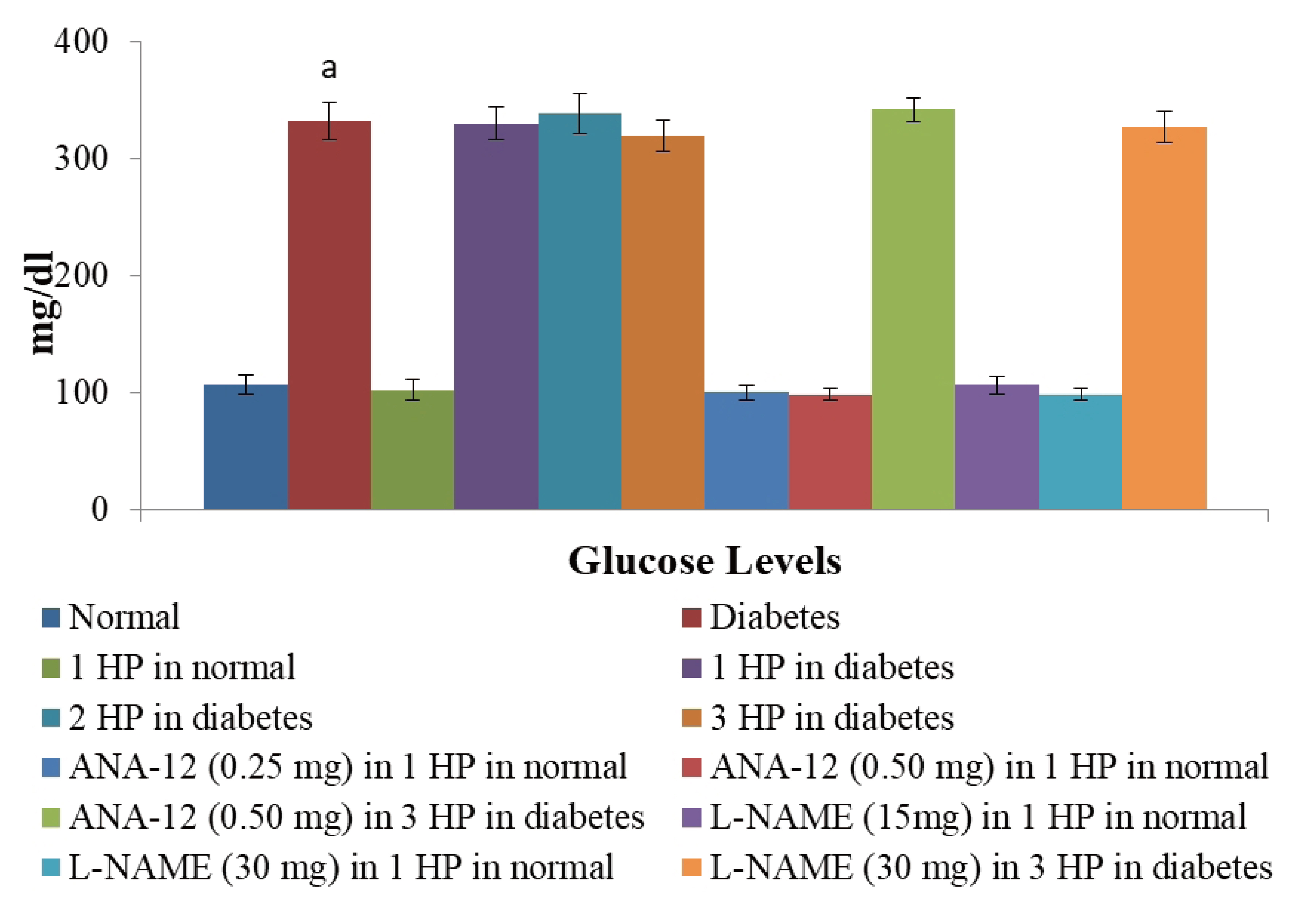
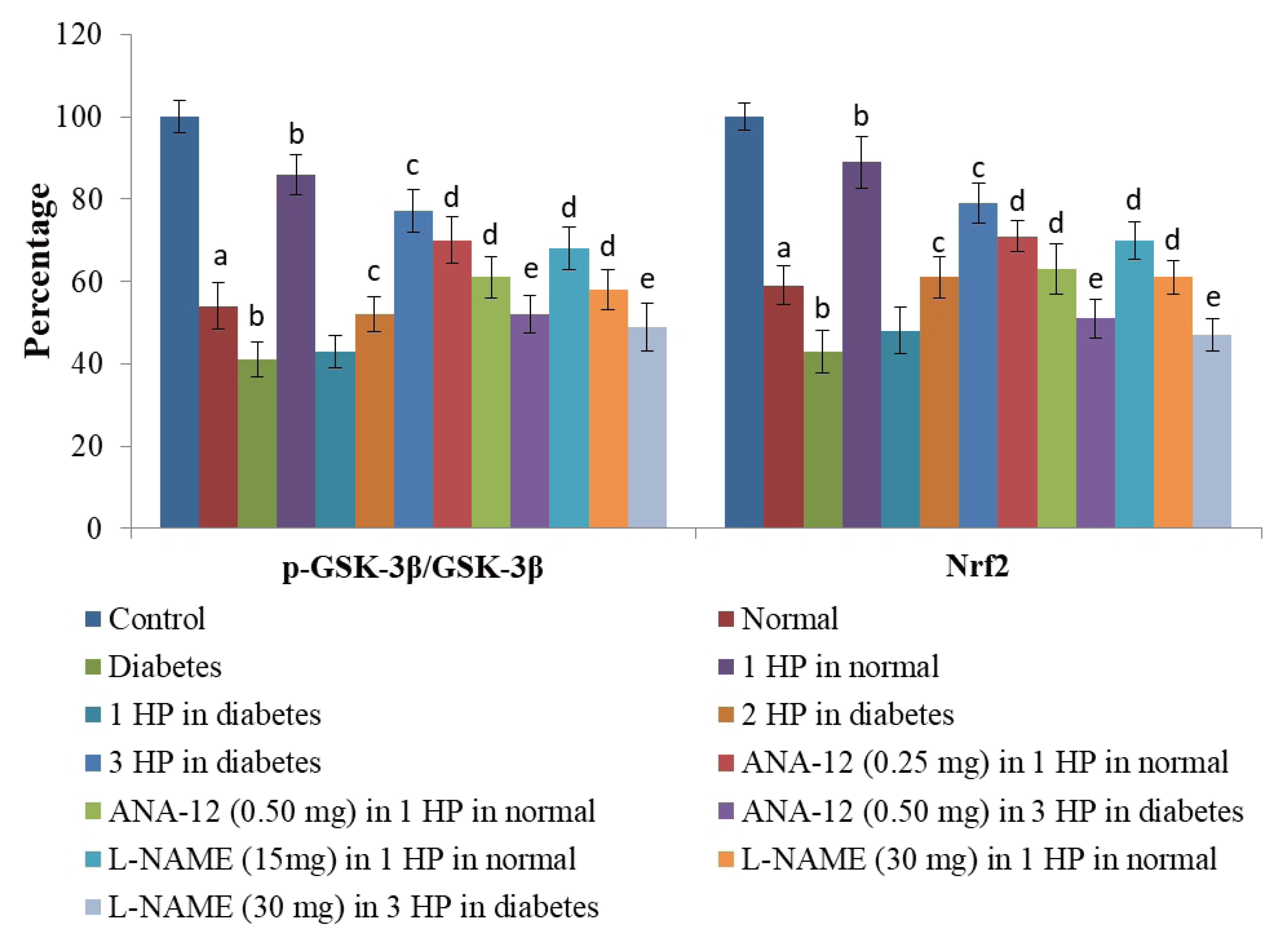
- The beneficial effects of hypoxic preconditioning are abolished in the diabetes. The present study was designed to investigate the protective effects and mechanisms of repeated episodes of whole body hypoxic preconditioning (WBHP) in db/db mice. The protective effects of preconditioning were explored on diabetesinduced vascular dysfunction, cognitive impairment and ischemia-reperfusion (IR)-induced increase in myocardial injury. Sixteen-week old db/db (diabetic) and C57BL/6 (non-diabetic) mice were employed. There was a significant impairment in cognitive function (Morris Water Maze test), endothelial function (acetylcholineinduced relaxation in aortic rings) and a significant increase in IR-induced heart injury (Langendorff apparatus) in db/db mice. WBHP stimulus was given by exposing mice to four alternate cycles of low (8%) and normal air O2 for 10 min each. A single episode of WBHP failed to produce protection; however, two and three episodes of WBHP significantly produced beneficial effects on the heart, brain and blood vessels. There was a significant increase in the levels of brain-derived neurotrophic factor (BDNF) and nitric oxide (NO) in response to 3 episodes of WBHP. Moreover, pretreatment with the BDNF receptor, TrkB antagonist (ANA-12) and NO synthase inhibitor (LNAME) attenuated the protective effects imparted by three episodes of WBHP. These pharmacological agents abolished WBHP-induced restoration of p-GSK-3β/GSK-3β ratio and Nrf2 levels in IR-subjected hearts. It is concluded that repeated episodes of WHBP attenuate cognitive impairment, vascular dysfunction and enhancement in IRinduced myocardial injury in diabetic mice be due to increase in NO and BDNF levels that may eventually activate GSK-3β and Nrf2 signaling pathway to confer protection.
Keyword
Figure
Reference
-
1. Sprick JD, Mallet RT, Przyklenk K, Rickards CA. 2019; Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol. 104:278–294. DOI: 10.1113/EP087122. PMID: 30597638. PMCID: PMC6397065.
Article2. Wu X, Liu X, Zhu X, Tang C. 2007; Hypoxic preconditioning induces delayed cardioprotection through p38 MAPK-mediated calreticulin upregulation. Shock. 27:572–577. DOI: 10.1097/01.shk.0000246901.58068.a8. PMID: 17438464.
Article3. Wang K, Xu BC, Duan HY, Zhang H, Hu FS. 2015; Late cardioprotection of exercise preconditioning against exhaustive exercise-induced myocardial injury by up-regulatation of connexin 43 expression in rat hearts. Asian Pac J Trop Med. 8:658–663. DOI: 10.1016/j.apjtm.2015.07.008. PMID: 26321521.
Article4. Hong XY, Hong X, Gu WW, Lin J, Yin WT. 2019; Cardioprotection and improvement in endothelial-dependent vasodilation during late-phase of whole body hypoxic preconditioning in spontaneously hypertensive rats via VEGF and endothelin-1. Eur J Pharmacol. 842:79–88. DOI: 10.1016/j.ejphar.2018.10.033. PMID: 30401629.
Article5. Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. 2013; Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013:653789. DOI: 10.1155/2013/653789. PMID: 23533715. PMCID: PMC3603160.
Article6. Liang H, Vallarino C, Joseph G, Manne S, Perez A, Zhang S. 2014; Increased risk of subsequent myocardial infarction in patients with type 2 diabetes: a retrospective cohort study using the U.K. General Practice Research Database. Diabetes Care. 37:1329–1337. DOI: 10.2337/dc13-1953. PMID: 24595635.
Article7. Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. 2013; Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 123:1262–1274. DOI: 10.1172/JCI65268. PMID: 23426181. PMCID: PMC3673230.
Article8. Schenning KJ, Anderson S, Alkayed NJ, Hutchens MP. 2015; Hyperglycemia abolishes the protective effect of ischemic preconditioning in glomerular endothelial cells in vitro. Physiol Rep. 3:e12346. DOI: 10.14814/phy2.12346. PMID: 25804266. PMCID: PMC4393174.9. Sasi M, Vignoli B, Canossa M, Blum R. 2017; Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 469:593–610. DOI: 10.1007/s00424-017-1964-4. PMID: 28280960. PMCID: PMC5438432.
Article10. Prakash YS, Martin RJ. 2014; Brain-derived neurotrophic factor in the airways. Pharmacol Ther. 143:74–86. DOI: 10.1016/j.pharmthera.2014.02.006. PMID: 24560686. PMCID: PMC3990251.
Article11. Türck P, Frizzo ME. 2015; Riluzole stimulates BDNF release from human platelets. Biomed Res Int. 2015:189307. DOI: 10.1155/2015/189307. PMID: 25629040. PMCID: PMC4300019.
Article12. Helan M, Aravamudan B, Hartman WR, Thompson MA, Johnson BD, Pabelick CM, Prakash YS. 2014; BDNF secretion by human pulmonary artery endothelial cells in response to hypoxia. J Mol Cell Cardiol. 68:89–97. DOI: 10.1016/j.yjmcc.2014.01.006. PMID: 24462831. PMCID: PMC3977651.
Article13. Walsh JJ, Tschakovsky ME. 2018; Exercise and circulating BDNF: mechanisms of release and implications for the design of exercise interventions. Appl Physiol Nutr Metab. 43:1095–1104. DOI: 10.1139/apnm-2018-0192. PMID: 29775542.
Article14. Neumann JT, Thompson JW, Raval AP, Cohan CH, Koronowski KB, Perez-Pinzon MA. 2015; Increased BDNF protein expression after ischemic or PKC epsilon preconditioning promotes electrophysiologic changes that lead to neuroprotection. J Cereb Blood Flow Metab. 35:121–130. DOI: 10.1038/jcbfm.2014.185. PMID: 25370861. PMCID: PMC4294405.
Article15. Zhen YF, Zhang J, Liu XY, Fang H, Tian LB, Zhou DH, Kosten TR, Zhang XY. 2013; Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology (Berl). 227:93–100. DOI: 10.1007/s00213-012-2942-3. PMID: 23263460.
Article16. Xie F, Rong B, Wang TC, Hao L, Lin MJ, Zhong JQ. 2017; Interaction between nitric oxide signaling and gap junctions during ischemic preconditioning: importance of S-nitrosylation vs. protein kinase G activation. Nitric Oxide. 65:37–42. DOI: 10.1016/j.niox.2017.02.003. PMID: 28216239.
Article17. Tessari P. 2015; Nitric oxide in the normal kidney and in patients with diabetic nephropathy. J Nephrol. 28:257–268. DOI: 10.1007/s40620-014-0136-2. PMID: 25216787.
Article18. Banoujaafar H, Monnier A, Pernet N, Quirié A, Garnier P, Prigent-Tessier A, Marie C. 2016; Brain BDNF levels are dependent on cerebrovascular endothelium-derived nitric oxide. Eur J Neurosci. 44:2226–2235. DOI: 10.1111/ejn.13301. PMID: 27306299.
Article19. McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F, Maestro R, Basecke J, Rakus D, Gizak A, Demidenko ZN, Cocco L, Martelli AM, Cervello M. 2014; GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 5:2881–2911. DOI: 10.18632/oncotarget.2037. PMID: 24931005. PMCID: PMC4102778.
Article20. Pall ML, Levine S. 2015; Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao. 67:1–18. PMID: 25672622.21. Yang T, Sun Y, Mao L, Zhang M, Li Q, Zhang L, Shi Y, Leak RK, Chen J, Zhang F. 2018; Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 17:323–337. DOI: 10.1016/j.redox.2018.05.001. PMID: 29775963. PMCID: PMC6007054.
Article22. Safaripour S, Nemati Y, Parvardeh S, Ghafghazi S, Fouladzadeh A, Moghimi M. 2018; Role of l-arginine/SNAP/NO/cGMP/KATP channel signalling pathway in antinociceptive effect of α-terpineol in mice. J Pharm Pharmacol. 70:507–515. DOI: 10.1111/jphp.12864. PMID: 29380385.
Article23. Ren Q, Zhang JC, Fujita Y, Ma M, Wu J, Hashimoto K. 2013; Effects of TrkB agonist 7,8-dihydroxyflavone on sensory gating deficits in mice after administration of methamphetamine. Pharmacol Biochem Behav. 106:124–127. DOI: 10.1016/j.pbb.2013.03.016. PMID: 23567202.
Article24. Vorhees CV, Williams MT. 2006; Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 1:848–858. DOI: 10.1038/nprot.2006.116. PMID: 17406317. PMCID: PMC2895266.
Article25. Bromley-Brits K, Deng Y, Song W. 2011; Morris water maze test for learning and memory deficits in Alzheimer's disease model mice. J Vis Exp. (53):2920. DOI: 10.3791/2920. PMID: 21808223. PMCID: PMC3347885.
Article26. Qiu F, Liu X, Zhang Y, Wu Y, Xiao D, Shi L. 2018; Aerobic exercise enhanced endothelium-dependent vasorelaxation in mesenteric arteries in spontaneously hypertensive rats: the role of melatonin. Hypertens Res. 41:718–729. DOI: 10.1038/s41440-018-0066-9. PMID: 29967417. PMCID: PMC6161704.
Article27. Bell RM, Mocanu MM, Yellon DM. 2011; Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. J Mol Cell Cardiol. 50:940–950. DOI: 10.1016/j.yjmcc.2011.02.018. PMID: 21385587.
Article28. Grünenfelder J, Miniati DN, Murata S, Falk V, Hoyt EG, Kown M, Koransky ML, Robbins RC. 2001; Upregulation of Bcl-2 through caspase-3 inhibition ameliorates ischemia/reperfusion injury in rat cardiac allografts. Circulation. 104(12 Suppl 1):I202–I206. DOI: 10.1161/hc37t1.094833. PMID: 11568056.
Article29. Zhang M, Gu WW, Hong XY. 2018; Involvement of endothelin 1 in remote preconditioning-induced cardioprotection through connexin 43 and Akt/GSK-3β signaling pathway. Sci Rep. 8:10941. DOI: 10.1038/s41598-018-29196-x. PMID: 30026513. PMCID: PMC6053397.
Article30. Miranda KM, Espey MG, Wink DA. 2001; A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5:62–71. DOI: 10.1006/niox.2000.0319. PMID: 11178938.
Article31. Feng X, Wang X, Liu Y, Di X. 2015; Linarin inhibits the acetylcholinesterase activity in-vitro and ex-vivo. Iran J Pharm Res. 14:949–954. PMID: 26330885. PMCID: PMC4518125.32. Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM. 1961; A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. DOI: 10.1016/0006-2952(61)90145-9. PMID: 13726518.
Article33. Burke SJ, Batdorf HM, Burk DH, Noland RC, Eder AE, Boulos MS, Karlstad MD, Collier JJ. 2017; db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a Western diet. J Diabetes Res. 2017:8503754. DOI: 10.1155/2017/8503754. PMID: 29038790. PMCID: PMC5606106.
Article34. Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. 2000; The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 49:22–31. DOI: 10.1016/S0026-0495(00)90588-2. PMID: 10647060.
Article35. Chen G, Fan XY, Zheng XP, Jin YL, Liu Y, Liu SC. 2020; Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem Cell Res Ther. 11:401. DOI: 10.1186/s13287-020-01865-7. PMID: 32938466. PMCID: PMC7493876.
Article36. Capiotti KM, De Moraes DA, Menezes FP, Kist LW, Bogo MR, Da Silva RS. 2014; Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav Brain Res. 274:319–325. DOI: 10.1016/j.bbr.2014.08.033. PMID: 25157430.
Article37. Thapak P, Bishnoi M, Sharma SS. 2020; Amelioration of diabetes-induced cognitive impairment by Transient Receptor Potential Vanilloid 2 (TRPV2) channel inhibitor: behavioral and mechanistic study. Neurochem Int. 139:104783. DOI: 10.1016/j.neuint.2020.104783. PMID: 32652268.
Article38. Zuloaga KL, Johnson LA, Roese NE, Marzulla T, Zhang W, Nie X, Alkayed FN, Hong C, Grafe MR, Pike MM, Raber J, Alkayed NJ. 2016; High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab. 36:1257–1270. DOI: 10.1177/0271678X15616400. PMID: 26661233. PMCID: PMC4929700.
Article39. Ma H, Jiang T, Tang W, Ma Z, Pu K, Xu F, Chang H, Zhao G, Gao W, Li Y, Wang Q. 2020; Transplantation of platelet-derived mitochondria alleviates cognitive impairment and mitochondrial dysfunction in db/db mice. Clin Sci. 134:2161–2175. DOI: 10.1042/CS20200530. PMID: 32794577.
Article40. Zhao D, Yang J, Yang L. 2017; Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev. 2017:6437467. DOI: 10.1155/2017/6437467. PMID: 28298952. PMCID: PMC5337354.
Article41. Bruder-Nascimento T, Callera GE, Montezano AC, He Y, Antunes TT, Nguyen Dinh Cat A, Tostes RC, Touyz RM. 2015; Vascular injury in diabetic db/db mice is ameliorated by atorvastatin: role of Rac1/2-sensitive Nox-dependent pathways. Clin Sci. 128:411–423. DOI: 10.1042/CS20140456. PMID: 25358739.
Article42. Russell JS, Griffith TA, Naghipour S, Vider J, Du Toit EF, Patel HH, Peart JN, Headrick JP. 2020; Dietary α-linolenic acid counters cardioprotective dysfunction in diabetic mice: unconventional PUFA protection. Nutrients. 12:E2679. DOI: 10.3390/nu12092679. PMID: 32887376. PMCID: PMC7551050.
Article43. Vinten-Johansen J, Shi W. 2011; Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 16:260–266. DOI: 10.1177/1074248411415270. PMID: 21821526.
Article44. Liu J, Zhan G, Chen D, Chen J, Yuan ZB, Zhang EL, Gao YX, Xu G, Sun BD, Liao W, Gao YQ. 2017; UPLC-QTOFMS-based metabolomic analysis of the serum of hypoxic preconditioning mice. Mol Med Rep. 16:6828–6836. DOI: 10.3892/mmr.2017.7493. PMID: 28901489. PMCID: PMC5865841.
Article45. Maslov LN, Lishmanov IuB, Kolar F, Portnichenko AG, Podoksenov IuK, Khaliulin IG, Wang H, Pei JM. 2010; Hypoxic preconditioning is a phenomenon increasing tolerance of cardiomyocytes to hypoxia-reoxygenation. Ross Fiziol Zh Im I M Sechenova. 96:1170–1189. Russian. PMID: 21473105.46. Shan D, Guo S, Wu HK, Lv F, Jin L, Zhang M, Xie P, Wang Y, Song Y, Wu F, Lan F, Hu X, Cao CM, Zhang Y, Xiao RP. 2020; Cardiac ischemic preconditioning promotes MG53 secretion through H2O2-activated protein kinase C-δ signaling. Circulation. 142:1077–1091. DOI: 10.1161/CIRCULATIONAHA.119.044998. PMID: 32677469.
Article47. Zhan L, Lu Z, Zhu X, Xu W, Li L, Li X, Chen S, Sun W, Xu E. 2019; Hypoxic preconditioning attenuates necroptotic neuronal death induced by global cerebral ischemia via Drp1-dependent signaling pathway mediated by CaMKIIα inactivation in adult rats. FASEB J. 33:1313–1329. DOI: 10.1096/fj.201800111RR. PMID: 30148677.48. Hong L, Sun Y, An JZ, Wang C, Qiao SG. 2020; Sevoflurane preconditioning confers delayed cardioprotection by upregulating AMP-activated protein kinase levels to restore autophagic flux in ischemia-reperfusion rat hearts. Med Sci Monit. 26:e922176. DOI: 10.12659/MSM.922176. PMID: 32476662. PMCID: PMC7288833.
Article49. Park KM, Chen A, Bonventre JV. 2001; Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 276:11870–11876. DOI: 10.1074/jbc.M007518200. PMID: 11150293.
Article50. Whittington HJ, Harding I, Stephenson CI, Bell R, Hausenloy DJ, Mocanu MM, Yellon DM. 2013; Cardioprotection in the aging, diabetic heart: the loss of protective Akt signalling. Cardiovasc Res. 99:694–704. DOI: 10.1093/cvr/cvt140. PMID: 23723063. PMCID: PMC3746954.
Article51. Vinokur V, Berenshtein E, Bulvik B, Grinberg L, Eliashar R, Chevion M. 2013; The bitter fate of the sweet heart: impairment of iron homeostasis in diabetic heart leads to failure in myocardial protection by preconditioning. PLoS One. 8:e62948. DOI: 10.1371/journal.pone.0062948. PMID: 23690966. PMCID: PMC3655153.
Article52. Lindsay A, Petersen C, Blackwell G, Ferguson H, Parker G, Steyn N, Gieseg SP. 2017; The effect of 1 week of repeated ischaemic leg preconditioning on simulated Keirin cycling performance: a randomised trial. BMJ Open Sport Exerc Med. 3:e000229. DOI: 10.1136/bmjsem-2017-000229. PMID: 28761713. PMCID: PMC5530127.
Article53. Rosenfeld RD, Zeni L, Haniu M, Talvenheimo J, Radka SF, Bennett L, Miller JA, Welcher AA. 1995; Purification and identification of brain-derived neurotrophic factor from human serum. Protein Expr Purif. 6:465–471. DOI: 10.1006/prep.1995.1062. PMID: 8527932.
Article54. Marie C, Pedard M, Quirié A, Tessier A, Garnier P, Totoson P, Demougeot C. 2018; Brain-derived neurotrophic factor secreted by the cerebral endothelium: a new actor of brain function? J Cereb Blood Flow Metab. 38:935–949. DOI: 10.1177/0271678X18766772. PMID: 29557702. PMCID: PMC5998997.
Article55. Supnick HT, Bunaciu RP, Yen A. 2018; The c-Raf modulator RRD-251 enhances nuclear c-Raf/GSK-3/VDR axis signaling and augments 1,25-dihydroxyvitamin D3-induced differentiation of HL-60 myeloblastic leukemia cells. Oncotarget. 9:9808–9824. DOI: 10.18632/oncotarget.24275. PMID: 29515772. PMCID: PMC5839403.
Article56. Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T. 2016; Repression of the antioxidant NRF2 pathway in premature aging. Cell. 165:1361–1374. DOI: 10.1016/j.cell.2016.05.017. PMID: 27259148. PMCID: PMC4893198.
Article57. Sharma AK, Kumar A, Sahu M, Sharma G, Datusalia AK, Rajput SK. 2018; Exercise preconditioning and low dose copper nanoparticles exhibits cardioprotection through targeting GSK-3β phosphorylation in ischemia/reperfusion induced myocardial infarction. Microvasc Res. 120:59–66. DOI: 10.1016/j.mvr.2018.06.003. PMID: 29940198.
Article58. Cadenas S. 2018; ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 117:76–89. DOI: 10.1016/j.freeradbiomed.2018.01.024. PMID: 29373843.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Supplementation of Clostridium butyricum Alleviates Vascular Inflammation in Diabetic Mice
- Niclosamide Inhibits Aortic Valve Interstitial Cell Calcification by Interfering with the GSK-3β/β-Catenin Signaling Pathway
- Effects of an Herbal Medicinal Product Composed of Three Herbal Materials on Lipopolysaccharide-induced Depression-like Behaviors in Mice
- Mechanism of Lipid Accumulation through PAR2 Signaling in Diabetic Male Mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation