Ann Rehabil Med.
2020 Dec;44(6):428-437. 10.5535/arm.20093.
Comparison of Repetitive Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation on Upper Limb Recovery Among Patients With Recent Stroke
- Affiliations
-
- 1Department of Rehabilitation and Extended Care, Wong Tai Sin Hospital, Hong Kong, China
- 2Department of Occupational Therapy, Wong Tai Sin Hospital, Hong Kong, China
- 3Department of Physiotherapy, Wong Tai Sin Hospital, Hong Kong, China
- KMID: 2510810
- DOI: http://doi.org/10.5535/arm.20093
Abstract
Objective
To compare the efficacy of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) on upper limb function recovery among patients who recently had stroke.
Methods
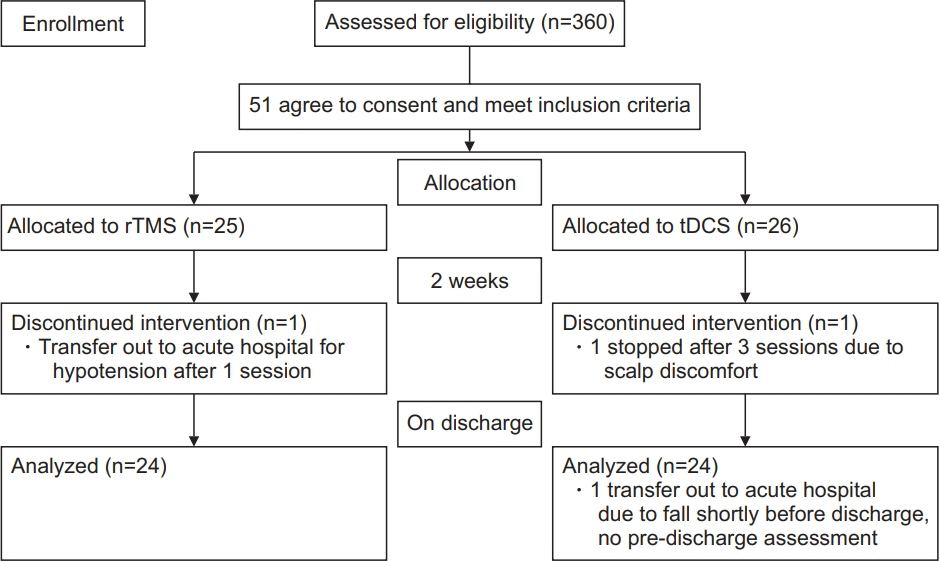
Subjects with recent stroke (within 1 month) were randomized to rTMS (n=25) and tDCS (n=26) applied over the non-lesioned hemisphere for three sessions per week, followed by tailored upper limb rehabilitation training for a total of 2 weeks. The primary outcomes were changes in the Motor Assessment Scale (MAS), Fugl-Meyer arm score test, Nine-Hole Peg Test (9HPT), hand grip strength, and modified Barthel Index at weeks 2 and 4. Both therapists responsible for training and assessment were blinded to the intervention allocated.
Results
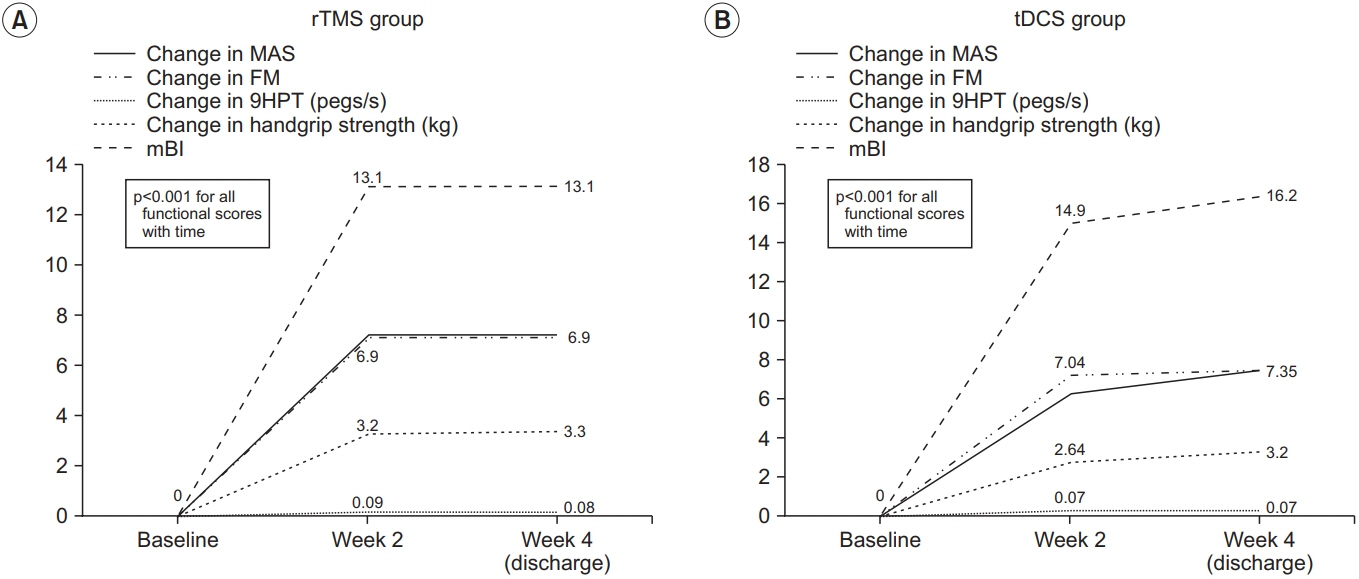
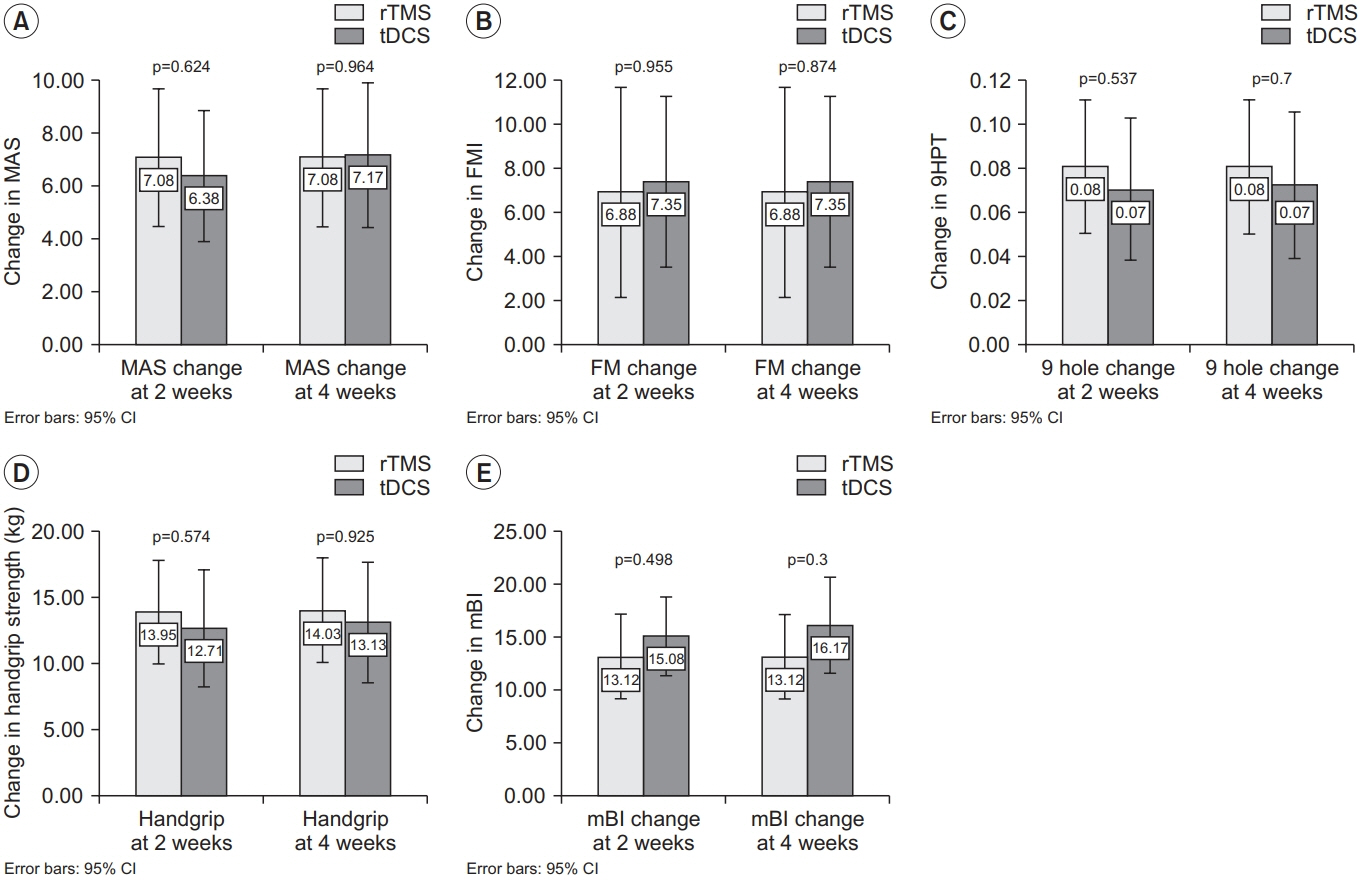
There was an improvement in all the motor performance scales among both groups (p<0.001). These improvements persisted at discharge. However, there was no significant difference in any of the assessment scales between the two groups. The rTMS group showed a statistically non-significant greater improvement in MAS, 9HPT, and handgrip strength than the tDCS group.
Conclusion
Both interventions produce a statistically significant improvement in upper limb function. There was no statistically significant difference between the two intervention methods with respect to motor performance. It is suggested that a larger study may help to clarify the superiority of either methods.
Keyword
Figure
Reference
-
1. Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005; 36:1480–4.
Article2. Broeks JG, Lankhorst GJ, Rumping K, Prevo AJ. The long-term outcome of arm function after stroke: results of a follow-up study. Disabil Rehabil. 1999; 21:357–64.3. Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke. 2003; 34:2181–6.4. Luke C, Dodd KJ, Brock K. Outcomes of the Bobath concept on upper limb recovery following stroke. Clin Rehabil. 2004; 18:888–98.
Article5. Kwakkel G, Wagenaar RC, Koelman TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke: a research synthesis. Stroke. 1997; 28:1550–6.6. Langhorne P, Wagenaar R, Partridge C. Physiotherapy after stroke: more is better? Physiother Res Int. 1996; 1:75–88.
Article7. Mansur CG, Fregni F, Boggio PS, Riberto M, GallucciNeto J, Santos CM, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005; 64:1802–4.
Article8. Goodwill AM, Teo WP, Morgan P, Daly RM, Kidgell DJ. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: a pilot study. Front Hum Neurosci. 2016; 10:258.
Article9. Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007; 55:187–99.
Article10. Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, et al. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol. 2014; 10:597–608.
Article11. Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009; 23:641–56.
Article12. Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006; 37:2115–22.
Article13. Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke: a double-blind placebocontrolled crossover trial. Restor Neurol Neurosci. 2007; 25:461–5.14. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008; 1:206–23.
Article15. Bastani A, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: a systematic review and meta-analysis. Clin Neurophysiol. 2012; 123:644–57.
Article16. Takeuchi N, Izumi S. Noninvasive brain stimulation for motor recovery after stroke: mechanisms and future views. Stroke Res Treat. 2012; 2012:584727.
Article17. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991; 337:1521–6.
Article18. Adams Jr HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial TOAST (Trial of Org 10172 in Acute Stroke Treatment). Stroke. 1993; 24:35–41.
Article19. Carr JH, Shepherd RB, Nordholm L, Lynne D. Investigation of a new motor assessment scale for stroke patients. Phys Ther. 1985; 65:175–80.
Article20. Ansari NN, Naghdi S, Arab TK, Jalaie S. The interrater and intrarater reliability of the Modified Ashworth Scale in the assessment of muscle spasticity: limb and muscle group effect. NeuroRehabilitation. 2008; 23:231–7.
Article21. Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983; 63:1606–10.
Article22. Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am J Occup Ther. 2003; 57:570–3.
Article23. Maeda F, Keenan JP, Tormos JM, Topka H, PascualLeone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000; 111:800–5.
Article24. Avenanti A, Coccia M, Ladavas E, Provinciali L, Ceravolo MG. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology. 2012; 78:256–64.
Article25. Nicolo P, Magnin C, Pedrazzini E, Plomp G, Mottaz A, Schnider A, et al. Comparison of neuroplastic responses to cathodal transcranial direct current stimulation and continuous theta burst stimulation in subacute stroke. Arch Phys Med Rehabil. 2018; 99:862–72.
Article26. Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and inputbias mechanisms. Front Hum Neurosci. 2013; 7:688.
Article27. Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol. 2014; 592:4115–28.
Article28. Tretriluxana J, Kantak S, Tretriluxana S, Wu AD, Fisher BE. Improvement in paretic arm reach-to-grasp following low frequency repetitive transcranial magnetic stimulation depends on object size: a pilot study. Stroke Res Treat. 2015; 2015:498169.
Article29. Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. The role of the human motor cortex in the control of complex and simple finger movement sequences. Brain. 1998; 121:1695–709.
Article30. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004; 55:400–9.
Article31. Lefebvre S, Dricot L, Laloux P, Gradkowski W, Desfontaines P, Evrard F, et al. Neural substrates underlying stimulation-enhanced motor skill learning after stroke. Brain. 2015; 138:149–63.
Article32. Bolognini N, Vallar G, Casati C, Latif LA, El-Nazer R, Williams J, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011; 25:819–29.
Article33. Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005; 36:2681–6.
Article34. Sergio LE, Hamel-Paquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol. 2005; 94:2353–78.
Article35. Haggard P, Wing A. Coordination of hand aperture with the spatial path of hand transport. Exp Brain Res. 1998; 118:286–92.
Article36. Opitz A, Paulus W, Will S, Antunes A, Thielscher A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 2015; 109:140–50.
Article37. Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010; 3:230–7.
Article38. Inukai Y, Saito K, Sasaki R, Tsuiki S, Miyaguchi S, Kojima S, et al. Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Front Hum Neurosci. 2016; 10:668.
Article39. Long H, Wang H, Zhao C, Duan Q, Feng F, Hui N, et al. Effects of combining high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. Restor Neurol Neurosci. 2018; 36:21–30.
Article40. Bornheim S, Croisier JL, Maquet P, Kaux JF. Transcranial direct current stimulation associated with physical-therapy in acute stroke patients: a randomized, triple blind, sham-controlled study. Brain Stimul. 2020; 13:329–36.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Non-invasive Brain Stimulation on Dysphagia after Stroke
- Stroke Update 2011: Stroke Rehabilitation
- Non-Invasive Brain Stimulation for Treatment of Focal Hand Dystonia: Update and Future Direction
- Noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation
- Repetitive Transcranial Magnetic Stimulation for Limb-Kinetic Apraxia in Parkinson's Disease