Cancer Res Treat.
2013 Mar;45(1):22-30.
Response Evaluation after Neoadjuvant Chemoradiation by Positron Emission Tomography-Computed Tomography for Esophageal Squamous Cell Carcinoma
- Affiliations
-
- 1Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ymshim@skku.edu
- 2Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Division of Hematology-Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Thoracic Surgery, Seoul National Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
Abstract
- PURPOSE
Parameters of positron emission tomography-computed tomography (PET-CT) were compared with the results of histopathologic examination in order to determine which can provide an objective indication of response after neoadjuvant chemoradiation for treatment of thoracic esophageal squamous cell carcinoma (SCC).
MATERIALS AND METHODS
Between August 2003 and January 2010, data on 25 patients who underwent neoadjuvant chemoradiation and subsequent resection for treatment of esophageal SCC were retrospectively reviewed. Changes in maximum standardized uptake value (DeltaSUVmax), metabolic tumor volume (DeltaMTV), and total lesion glycolysis (DeltaTLG) were analyzed by comparison with the histopathologic findings.
RESULTS
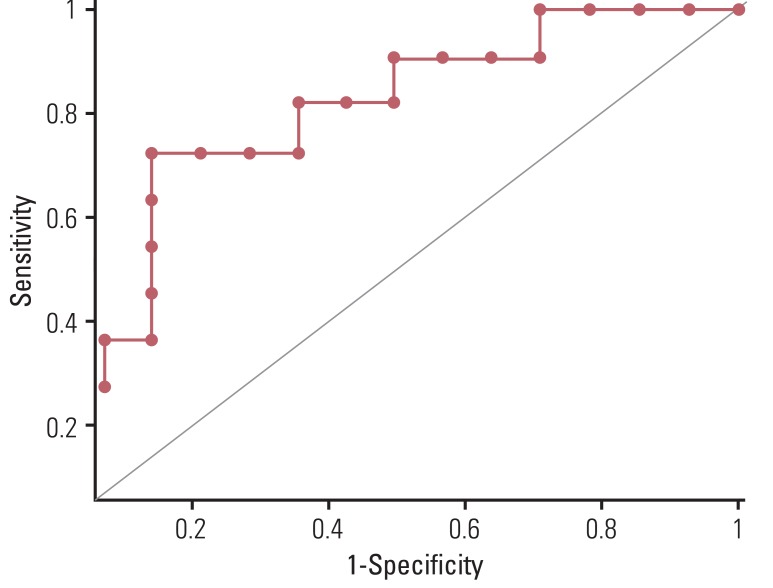
Pathologic complete remission (CR) for the main tumor was achieved in 11 patients. Postradiation esophagitis was observed in 10 patients. DeltaSUVmax of the main tumor was significantly greater in the CR group than in the partial response (PR) group (p=0.039), while DeltaMTV and DeltaTLG of the main tumor were not (p=0.141 and p=0.349, respectively). The cut-off DeltaSUVmax value for CR was estimated as 72.1%, indicating significantly better accuracy than visual interpretation (p=0.045). Of the 48 involved lymph nodes, DeltaSUVmax and DeltaMTV of lymph nodes were significantly greater in the CR group than in the PR group (p=0.045 and p=0.014, respectively), while DeltaTLG was not (p=0.063). The cut-off value of DeltaSUVmax for prediction of CR in lymph nodes was calculated as 50.67%.
CONCLUSION
PET-CT could be used for prediction of response to neoadjuvant treatment in thoracic esophageal SCC. DeltaSUVmax may be a more significant predictor for CR after neoadjuvant chemoradiation than DeltaTLG and DeltaMTV.
Keyword
MeSH Terms
Figure
Reference
-
1. Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg. 2001; 88:338–356. PMID: 11260097.
Article2. Poplin E, Fleming T, Leichman L, Seydel HG, Steiger Z, Taylor S, et al. Combined therapies for squamous-cell carcinoma of the esophagus, a Southwest Oncology Group Study (SWOG-8037). J Clin Oncol. 1987; 5:622–628. PMID: 3559653.
Article3. Bates BA, Detterbeck FC, Bernard SA, Qaqish BF, Tepper JE. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol. 1996; 14:156–163. PMID: 8558191.
Article4. Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996; 335:462–467. PMID: 8672151.
Article5. Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001; 19:305–313. PMID: 11208820.
Article6. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011; 12:681–692. PMID: 21684205.
Article7. Zuccaro G Jr, Rice TW, Goldblum J, Medendorp SV, Becker M, Pimentel R, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol. 1999; 94:906–912. PMID: 10201455.
Article8. Jones DR, Parker LA Jr, Detterbeck FC, Egan TM. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer. 1999; 85:1026–1032. PMID: 10091784.
Article9. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008; 49:480–508. PMID: 18287273.
Article10. Brucher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001; 233:300–309. PMID: 11224616.11. Mamede M, Abreu-E-Lima P, Oliva MR, Nose V, Mamon H, Gerbaudo VH. FDG-PET/CT tumor segmentation-derived indices of metabolic activity to assess response to neoadjuvant therapy and progression-free survival in esophageal cancer: correlation with histopathology results. Am J Clin Oncol. 2007; 30:377–388. PMID: 17762438.12. Sendler A. Metabolic response evaluation by PET during neoadjuvant treatment for adenocarcinoma of the esophagus and esophagogastric junction. Recent Results Cancer Res. 2010; 182:167–177. PMID: 20676880.
Article13. Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT: comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008; 89:278–286. PMID: 18701180.14. Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010; 17:115–122. PMID: 19826877.15. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007; 25:571–578. PMID: 17242397.
Article16. Le Roux PY, Gastinne T, Le Gouill S, Nowak E, Bodet-Milin C, Querellou S, et al. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging. 2011; 38:1064–1071. PMID: 21308370.
Article17. van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010; 20:1456–1467. PMID: 20033179.
Article18. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer. 1999; 35:1773–1782. PMID: 10673991.19. Thurau K, Palmes D, Franzius C, Minin E, Senninger N, Juergens KU, et al. Impact of PET-CT on primary staging and response control on multimodal treatment of esophageal cancer. World J Surg. 2011; 35:608–616. PMID: 21221582.
Article20. Kim JH, Choi EK, Kim SB, Park SI, Kim DK, Song HY, et al. Preoperative hyperfractionated radiotherapy with concurrent chemotherapy in resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2001; 50:1–12. PMID: 11316540.
Article21. van Heijl M, Omloo JM, van Berge Henegouwen MI, Hoekstra OS, Boellaard R, Bossuyt PM, et al. Fluorodeoxyglucose positron emission tomography for evaluating early response during neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer. Ann Surg. 2011; 253:56–63. PMID: 21233607.
Article22. Luu TD, Gaur P, Force SD, Staley CA, Mansour KA, Miller JI Jr, et al. Neoadjuvant chemoradiation versus chemotherapy for patients undergoing esophagectomy for esophageal cancer. Ann Thorac Surg. 2008; 85:1217–1223. PMID: 18355499.
Article23. Aiko S, Yoshizumi Y, Ishizuka T, Sakano T, Kumano I, Sugiura Y, et al. Reduction rate of lymph node metastasis as a significant prognostic factor in esophageal cancer patients treated with neoadjuvant chemoradiation therapy. Dis Esophagus. 2007; 20:94–101. PMID: 17439591.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Use of PET in Esophageal Cancer
- Long-term survival after concurrent chemoradiation therapy for esophageal cancer with tracheal invasion
- Fluorodeoxyglucose-positron emission tomography/computed tomography imaging of squamous cell carcinoma arising in a meningomyelocele
- Basic principles and applications of 18F-FDG-PET/CT in oral and maxillofacial imaging: A pictorial essay
- PET Imaging for Gynecologic Malignancy