J Rhinol.
2020 Nov;27(2):108-116. 10.18787/jr.2020.00325.
Bacillus clausii, a Foreshore-Derived Probiotic, Attenuates Allergic Airway Inflammation Through Downregulation of Hypoxia Signaling
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Inha University College of Medicine, Incheon, Korea
- 2Inha Institute of Aerospace Medicine, Inha University College of Medicine, Incheon, Korea
- 3Inha Institute of Biochemistry, Inha University College of Medicine, Incheon, Korea
- KMID: 2508955
- DOI: http://doi.org/10.18787/jr.2020.00325
Abstract
- Background and Objectives
The immunomodulatory effects and mechanism of probiotics in allergic airway disease are largely unknown. We studied whether Bacillus clausii (BC), a probiotic derived from mudflats, had anti-allergic effects and compared the results with those of Lactobacillus paracasei (LP). We also examined whether the anti-allergic mechanisms of probiotics are associated with hypoxia signaling. Materials and Method: Forty-two BALB/c mice were randomly assigned to six experimental groups: controls, ovalbumin (OVA)-induced mice for inducing asthma, and OVA-induced mice that were orally administered LP or BC, at 1×109 or 5×109 CFU/mL each. We performed differential cell count testing on bronchoalveolar lavage fluid (BALF), lung histopathology, serum totals and OVA-specific IgE and IgG1 assessments, Th2 cytokine titers (IL-4, IL-5) in BALF and pulmonary parenchyma, quantitative PCR for heme oxygenase (HO)-1 and Hif-1α, and immunohistochemistry.
Results
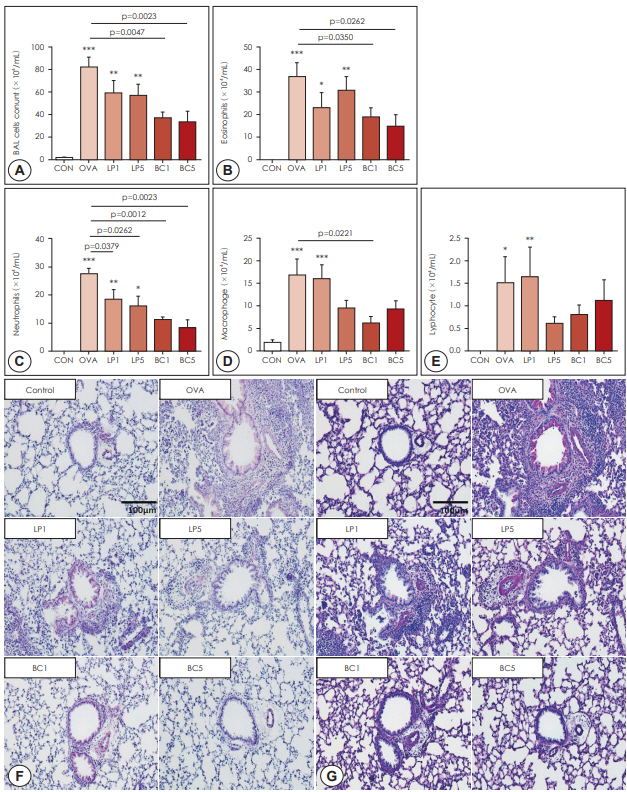
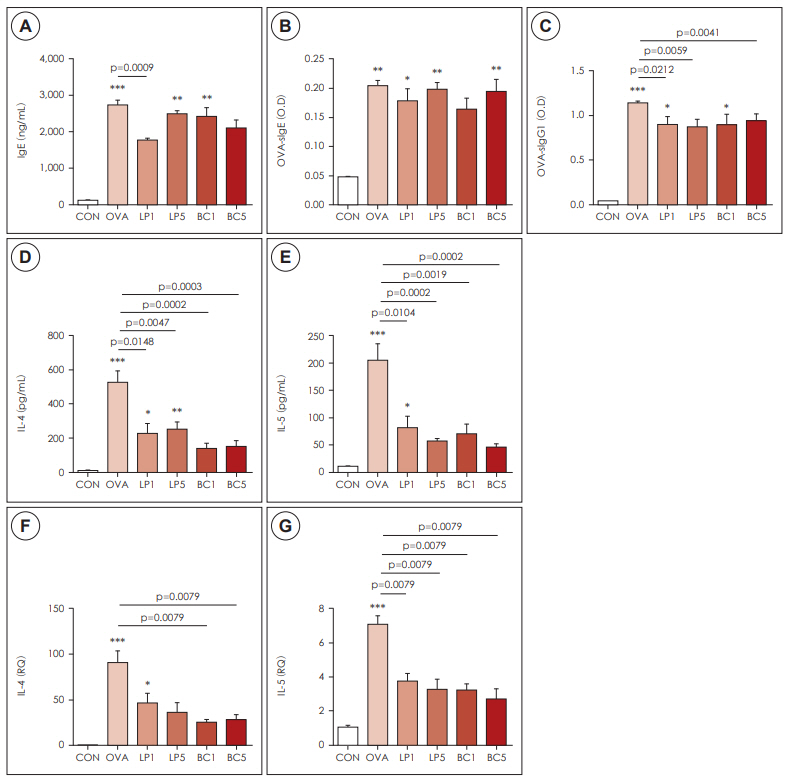
Compared to the OVA group mice, OVA-sensitized mice treated with LP or BC showed significantly reduced numbers of eosinophils and neutrophils in the BALF (p<0.05). Both probiotics also significantly reduced pulmonary inflammation and eosinophil infiltration. Mice in the LP or BC group had a substantially lower titer of IL-4 and IL-5 in BALF, and decreased IL-4 and IL-5 expression in the lung parenchyma. Real-time PCR and immunohistochemistry showed that both LP and BC could significantly suppress HO-1 and Hif-1α expression in asthmatic mice (p<0.05).
Conclusion
BC can attenuate murine allergic asthma by regulating HIF-1α signaling, and its anti-allergic effect is comparable to that of LP.
Figure
Reference
-
References
1. Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013; 12:117–29.2. Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011; 128:495–505.3. Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001; 1:69–75.4. Christodoulopoulos P, Cameron L, Durham S, Hamid Q. Molecular pathology of allergic disease. II: Upper airway disease. J Allergy Clin Immunol. 2000; 105:211–23.5. Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, Dohil R, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol. 2013; 131:1576–82.6. Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005; 116:961–8. quiz 9.7. Ozdemir O. Various effects of different probiotic strains in allergic disorders: an update from laboratory and clinical data. Clin Exp Immunol. 2010; 160:295–304.8. Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001; 357:1076–9.9. Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus clausii exerts immuno-modulatory activity in allergic subjects: a pilot study. Eur Ann Allergy Clin Immunol. 2005; 37:129–34.10. Fatani SH. Biomarkers of oxidative stress in acute and chronic bronchial asthma. J Asthma. 2014; 51:578–84.11. Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003; 22:3898–909.12. Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, et al. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol. 2002; 72:215–24.13. Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007; 37:498–505.14. Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007; 175:561–9.15. Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992; 326:298–304.16. Elson CO, Cong Y. Understanding immune-microbial homeostasis in intestine. Immunol Res. 2002; 26:87–94.17. Ciprandi G, Vizzaccaro A, Cirillo I, Tosca MA. Bacillus clausii effects in children with allergic rhinitis. Allergy. 2005; 60:702–3.18. Wang X, Hui Y, Zhao L, Hao Y, Guo H, Ren F. Oral administration of Lactobacillus paracasei L9 attenuates PM2.5-induced enhancement of airway hyperresponsiveness and allergic airway response in murine model of asthma. PLoS One. 2017; 12:e0171721.19. Saini Y, Greenwood KK, Merrill C, Kim KY, Patial S, Parameswaran N, et al. Acute cobalt-induced lung injury and the role of hypoxiainducible factor 1alpha in modulating inflammation. Toxicol Sci. 2010; 116:673–81.20. Saini Y, Kim KY, Lewandowski R, Bramble LA, Harkema JR, Lapres JJ. Role of hypoxia-inducible factor 1{alpha} in modulating cobalt-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2010; 298:L139–47.21. zmijewski jw, lorne e, zhao x, tsuruta y, sha y, liu g, et al. mitochondrial respiratory complex i regulates neutrophil activation and severity of lung injury. am j respir crit care med. 2008; 178:168–79.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TRIF Deficiency does not Affect Severity of Ovalbumin-induced Airway Inflammation in Mice
- Application of Stem Cell-Derived Extracellular Vesicles in Allergic Airway Diseases
- A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha
- Airway epithelial cells in airway inflammation and remodeling in asthma
- Stem cell therapy in animal models of allergic airway diseases