J Rhinol.
2020 May;27(1):1-7. 10.18787/jr.2020.00312.
Application of Stem Cell-Derived Extracellular Vesicles in Allergic Airway Diseases
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Pusan National University School of Medicine, Pusan National University Hospital, Busan, Korea
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- KMID: 2502787
- DOI: http://doi.org/10.18787/jr.2020.00312
Abstract
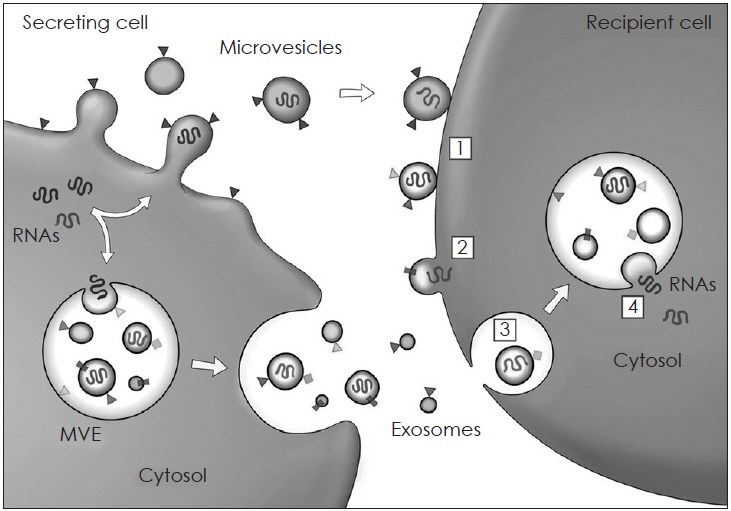
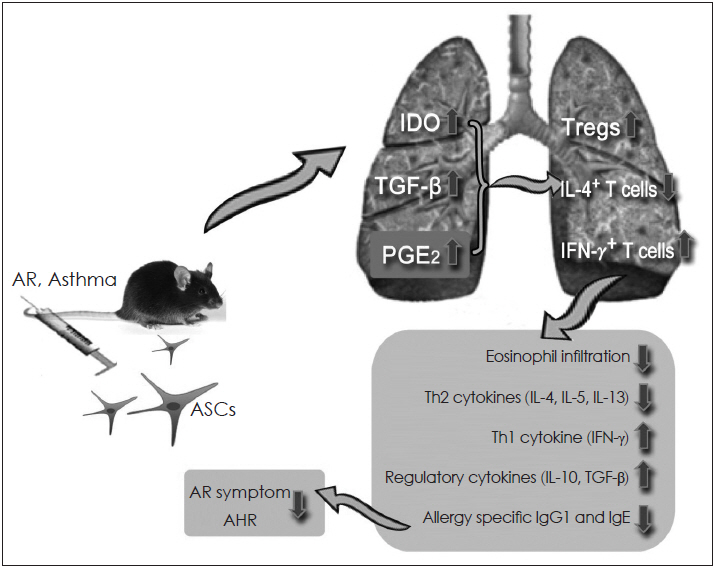
- Mesenchymal stem cells (MSCs) have been reported to be promising candidates for the treatment of allergic airway diseases. However, MSCs themselves have several problems including immune rejection, risk of aneuploidy, difficulty of handling, and tumorigenicity. An increasing number of studies demonstrated that administration of conditioned media or extracellular vesicles (EVs) released by MSCs is as effective as the MSCs themselves in suppression of allergic airway inflammation. EVs can exert their effects by delivering their contents such as proteins, mRNAs, and microRNAs to recipient cells. Furthermore, the administration of MSCs-derived EVs may reduce potential safety risks associated with stem cell therapy, suggesting that MSCs-derived EVs may be a promising alternative to cell therapy for allergic airway diseases. This review examines the current understanding of the immunomodulatory properties of MSCs-derived EVs and its therapeutic implication for allergic airway diseases.
Keyword
Figure
Reference
-
1. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immumol. 2008; 8:726–62.2. Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003; 5:32–45.3. Law S, Chaudhuri S. Mesenchymal stem cell and regenerative medicine: regenerative versus immunomodulatory challenges. Am J Stem Cells. 2013; 8:22–38.4. Cho KS. Stem cell therapy in animal models of allergic airway diseases. Allergy Asthma Respir Dis. 2016; 4:167–73.5. Cho KS. Application of mesenchymal stem cells in rhinologic fields. Korean J Otorhinolaryngol-Head Neck Surg. 2014; 57:207–13.6. Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007; 140:6781.7. Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007; 25:750–60.8. Titorencu I, Jinga V, Constantinescu E, Gafencu A, Ciohodaru C, Manolescu I, et al. Proliferation, differentiation and characterization of osteoblasts from human BM mesenchymal cells. Cytotherapy. 2007; 9:682–96.9. Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004; 318:119–24.10. Shi M, Liu ZW, Wang FS. Imunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011; 164:1–8.11. Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014; 21:216–25.12. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106:1755–61.13. Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009; 15:42–9.14. Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006; 103:17438–43.15. Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004; 363:1439–41.16. Kim HS, Shin TH, Lee BC, Yu KR, Seo Y, Lee S, et al. Human umbilical cord blood mesenchymal stem cells reduce colitis in mice by activating NOD2 signaling to COX2. Gastroenterology. 2013; 145:1392–403.17. González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009; 136:978–89.18. Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007; 56:1175–86.19. Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells. 2012; 30:2692–9.20. Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Bundoc VG, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc Natl Acad Sci U S A. 2010; 107:5652–7.21. Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells. 2011; 29:1137–48.22. Cho KS, Park HK, Park HY, Jung JS, Jeon SG, Kim YK, et al. IFATS collection: immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model. Stem Cells. 2009; 27:259–65.23. Cho KS, Roh HJ. Immunomodulatory effects of adipose-derived stem cells in airway allergic diseases. Curr Stem Cell Res Ther. 2010; 5:111–5.24. Park HK, Cho KS, Park HY, Shin DH, Kim YK, Jung JS, et al. Adipose-derived stromal cells inhibit allergic airway inflammation in mice. Stem Cells Dev. 2010; 19:1811–8.25. Cho KS, Park MK, Kang S, Park HY, Hong SL, Park HK, et al. Adipose-derived stem cells ameliorate allergic airway inflammation by inducing regulatory T cells in a mouse model of asthma. Mediators Inflamm. 2014; 2014:436476.26. Trohatou O, Roubelakis MG. Mesenchymal stem/stromal cells in regenerative medicine: past, present, and future. Cell Reprogram. 2017; 19:217–24.27. Yang YK. Aging of mesenchymal stem cells: implication in regenerative medicine. Regen Ther. 2018; 9:120–2.28. Gyorgy B, Szabo TG, Pasztoi M, Pai Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011; 68:2667–88.29. Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekstrom K, Wang X, et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol. 2014; 11:688–701.30. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013; 200:373–83.31. Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010; 73:1907–20.32. Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006; 20:1487–95.33. Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018; 3:99263.34. Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017; 6:1324730.35. Ingato D, Lee JU, Sim SJ, Kwon YJ. Good things come in small packages: overcoming challenges to harness extracellular vesicles for therapeutic delivery. J Control Release. 2016; 241:174–85.36. Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: new nanotools for cancer treatment. Pharmacol Res. 2016; 111:487–500.37. Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017; 38:754–63.38. Boulay ME, Boulet LP. The relationships between atopy, rhinitis and asthma: pathophysiological considerations. Curr Opin Allergy Clin Immunol. 2003; 3:51–5.39. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003; 111:1171–83.40. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005; 202:1199–212.41. Shi HZ, Qin XJ. CD4CD25 regulatory T lymphocytes in allergy and asthma. Allergy. 2005; 60:986–95.42. Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004; 172:3842–9.43. Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB, Shi JB, et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012; 67:121522.44. Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Intratracheal transplantation of bone marrow-derived mesenchymal stem cells reduced airway inflammation and up-regulated CD4+CD25+ regulatory T cells in asthmatic mouse. Cell Biol Int. 2013; 37:675–86.45. Ge X, Bai C, Yang J, Lou G, Li Q, Chen R. Effect of mesenchymal stem cells on inhibiting airway remodeling and airway inflammation in chronic asthma. J Cell Biochem. 2013; 114:1595–605.46. Cui L, Yin S, Liu W, Li N, Zhang W, Cao Y. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007; 13(6):1185–95.47. Cho KS, Lee JH, Park MK, Park HK, Yu HS, Roh HJ. Prostaglandin E2 and Transforming Growth Factor-β play a critical role in suppression of allergic airway inflammation by adipose-derived stem cells. PLos One. 2015; 10:e0131813.48. Cho KS, Park MK, Mun SJ, Park HY, Yu HS, Roh HJ. Indoleamine 2,3-Dioxygenase is not a pivotal regulator responsible for suppressing allergic airway inflammation through adipose-derived stem cells. PLos One. 2016; 11:e0165661.49. Yu HS, Park MK, Kang SA, Cho KS, Mun SJ, Roh HJ. Culture supernatant of adipose stem cells can ameliorate allergic airway inflammation via recruitment of CD4+CD25+Foxp3 T cells. Stem Cell Res Ther. 2017; 8:8.50. Cruz FF, Borg ZD, Goodwin M, Sokocevic D, Wagner DE, Coffey A, et al. Systemic administration of human bone marrow derived mesenchymal stromal cell extracellular vesicles ameliorates aspergillus hyphal extract-induced allergic airway inflammation in immunocompetent mice. Stem Cells Transl Med. 2015; 4:1302–16.51. de Castro LL, Xisto DG, Kitoko JZ, Cruz FF, Olsen PC, Redondo PAG, et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Rest Ther. 2017; 8:151.52. Cho KS, Kang SA, Kim SD, Mun SJ, Yu HS, Roh HJ. Dendritic cells and M2 macrophage play an important role in suppression of Th2-mediated inflammation by adipose stem cells-derived extracellular vesicles. Stem Cell Res. 2019; 39:101500.53. Seo Y, Kim HS, Hong IS. Stem cell-derived extracellular vesicles as immunomodulatory therapeutics. Stem Cells Int. 2019; 2019:5126156.54. Eirin A, Riester SM, Zhu XY, Tang H, Evans JM, O’Brien D, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014; 551:5564.55. Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013; 22:772–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic potential of stem cell-derived extracellular vesicles in osteoarthritis: preclinical study findings
- Extracellular Vesicles Derived from Mesenchymal Stem Cells as Cell-Free Therapy for Intrauterine Adhesion
- Extracellular Vesicles and Immune System in Ageing and Immune Diseases
- Secretome of Stem Cells: Roles of Extracellular Vesicles in Diseases, Stemness, Differentiation, and Reprogramming
- Stem cell therapy in animal models of allergic airway diseases