Cancer Res Treat.
2020 Jul;52(3):671-679. 10.4143/crt.2019.387.
Comparison of the Distribution Pattern of 21-Gene Recurrence Score between Mucinous Breast Cancer and Infiltrating Ductal Carcinoma in Chinese Population: A Retrospective Single-Center Study
- Affiliations
-
- 1Comprehensive Breast Health Center, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Clinical Laboratory, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- KMID: 2504449
- DOI: http://doi.org/10.4143/crt.2019.387
Abstract
- Purpose
This retrospective study aimed to evaluate the distribution pattern and prognostic value of 21-gene recurrence score (RS) in Chinese patients with mucinous breast cancer (MC) and compared with infiltrating ductal carcinoma (IDC).
Materials and Methods
Patients diagnosed with MC or IDC from January 2010 to January 2017 were retrospectively recruited. Reverse transcriptase–polymerase chain reaction assay of 21 genes was conducted to calculate the RS. Univariate and multivariate analyses were performed to assess the association between RS and clinicopathological factors. Survival outcomes including disease-free survival (DFS) and overall survival (OS) were estimated by Kaplan-Meier method and compared by log-rank test.
Results
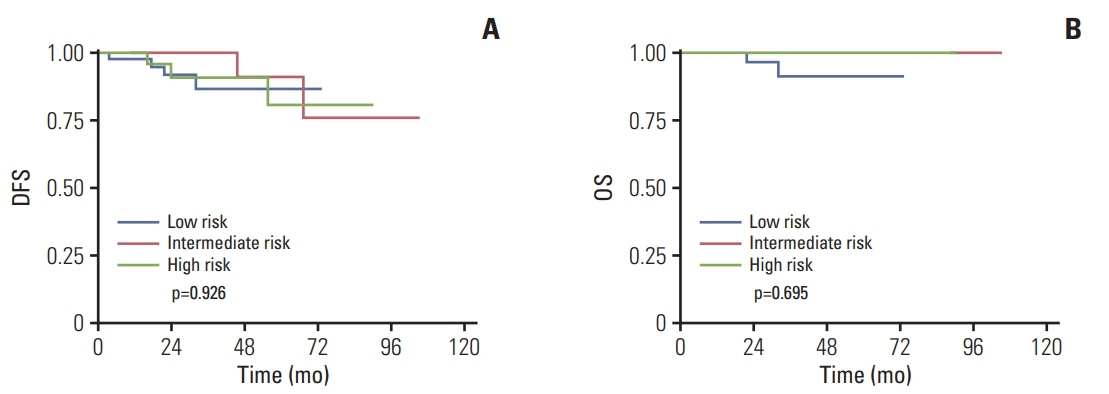
The MC cohort included 128 patients and the IDC cohort included 707 patients. The proportions of patients with a low (RS < 18), intermediate (18-30), or high risk (RS > 30) were 32.0%, 48.4%, and 19.5% in MC cohort, and 26.9%, 46.8% and 26.3% in IDC cohort. The distribution of RS varied significantly according to different Ki-67 index and molecular subtype in both cohorts. Moreover, the receipt of chemotherapy was associated with RS in both cohorts. Among patients with MC, tumor stage was related to the DFS (p=0.040). No significant differences in DFS and OS were found among MC patients in different RS risk groups (OS, p=0.695; DFS, p=0.926).
Conclusion
RS was significantly related to Ki-67 index and molecular subtypes in MC patients, which is similar in IDC patients. However, RS was not able to predict DFS and OS in patients with MC.
Figure
Reference
-
References
1. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004; 351:2817–26.
Article2. Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010; 28:1829–34.
Article3. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with nodenegative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006; 24:3726–34.
Article4. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010; 11:55–65.
Article5. Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y. Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer. 1988; 61:989–96.6. Andre S, Cunha F, Bernardo M, Meneses e Sousa J, Cortez F, Soares J. Mucinous carcinoma of the breast: a pathologic study of 82 cases. J Surg Oncol. 1995; 58:162–7.7. Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999; 17:1442–8.
Article8. Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005; 93:1046–52.
Article9. Louwman MW, Vriezen M, van Beek MW, Nolthenius-Puylaert MC, van der Sangen MJ, Roumen RM, et al. Uncommon breast tumors in perspective: incidence, treatment and survival in the Netherlands. Int J Cancer. 2007; 121:127–35.
Article10. Park S, Koo J, Kim JH, Yang WI, Park BW, Lee KS. Clinicopathological characteristics of mucinous carcinoma of the breast in Korea: comparison with invasive ductal carcinomanot otherwise specified. J Korean Med Sci. 2010; 25:361–8.
Article11. Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010; 63:1043–7.
Article12. Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011; 14:308–13.
Article13. Hanagiri T, Ono K, Baba T, So T, Yamasaki M, Nagata Y, et al. Clinicopathologic characteristics of mucinous carcinoma of the breast. Int Surg. 2010; 95:126–9.14. Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008; 196:549–51.
Article15. Wu J, Fang Y, Lin L, Fei X, Gao W, Zhu S, et al. Distribution patterns of 21-gene recurrence score in 980 Chinese estrogen receptor-positive, HER2-negative early breast cancer patients. Oncotarget. 2017; 8:38706–16.
Article16. Wang W, Chen X, Lin L, Fei X, Garfield DH, Hong J, et al. Distribution and clinical utility of the 21-gene recurrence score in pure mucinous breast cancer patients: a case-control study. J Cancer. 2018; 9:3216–24.
Article17. Chen X, Zhu S, Fei X, Garfield DH, Wu J, Huang O, et al. Surgery time interval and molecular subtype may influence Ki67 change after core needle biopsy in breast cancer patients. BMC Cancer. 2015; 15:822.
Article18. Zong Y, Zhu L, Wu J, Chen X, Huang O, Fei X, et al. Progesterone receptor status and Ki-67 index may predict early relapse in luminal B/HER2 negative breast cancer patients: a retrospective study. PLoS One. 2014; 9:e95629.
Article19. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–95.
Article20. Kizy S, Huang JL, Marmor S, Blaes A, Yuan J, Beckwith H, et al. Distribution of 21-gene recurrence scores among breast cancer histologic subtypes. Arch Pathol Lab Med. 2018; 142:735–41.
Article21. Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M, et al. West German study Group Phase III PlanB Trial: first prospective outcome data for the 21-gene recurrence score assay and concordance of prognostic markers by central and local pathology assessment. J Clin Oncol. 2016; 34:2341–9.
Article22. Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008; 111:541–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiographic Characteristics of Male Breast Cancer
- Clinicopathologic Analysis of 40 Mucinous Breast Carcinomas
- MR Imaging of Mucinous Carcinoma of the Breast Associated with Ductal Carcinoma In Situ: Case Report
- Mucocele-Like Tumor of the Breast Associated with Ductal Carcinoma In Situ and Mucinous Carcinoma : A Case Report
- Gd-DTPA Enhanced Dynamic IVIRI of the Breast Cancer