Korean J Physiol Pharmacol.
2020 Jul;24(4):299-310. 10.4196/kjpp.2020.24.4.299.
Combination therapy with cilostazol, aripiprazole, and donepezil protects neuronal cells from β-amyloid neurotoxicity through synergistically enhanced SIRT1 expression
- Affiliations
-
- 1Department of Pharmacology, Pusan National University School of Medicine, Korea
- 2Gene & Cell Therapy Research Center for Vessel-associated Diseases, Pusan National University, Korea
- 3Department of Korean Medical Science, Pusan National University School of Korean Medicine, Yangsan 50612, Korea
- KMID: 2503323
- DOI: http://doi.org/10.4196/kjpp.2020.24.4.299
Abstract
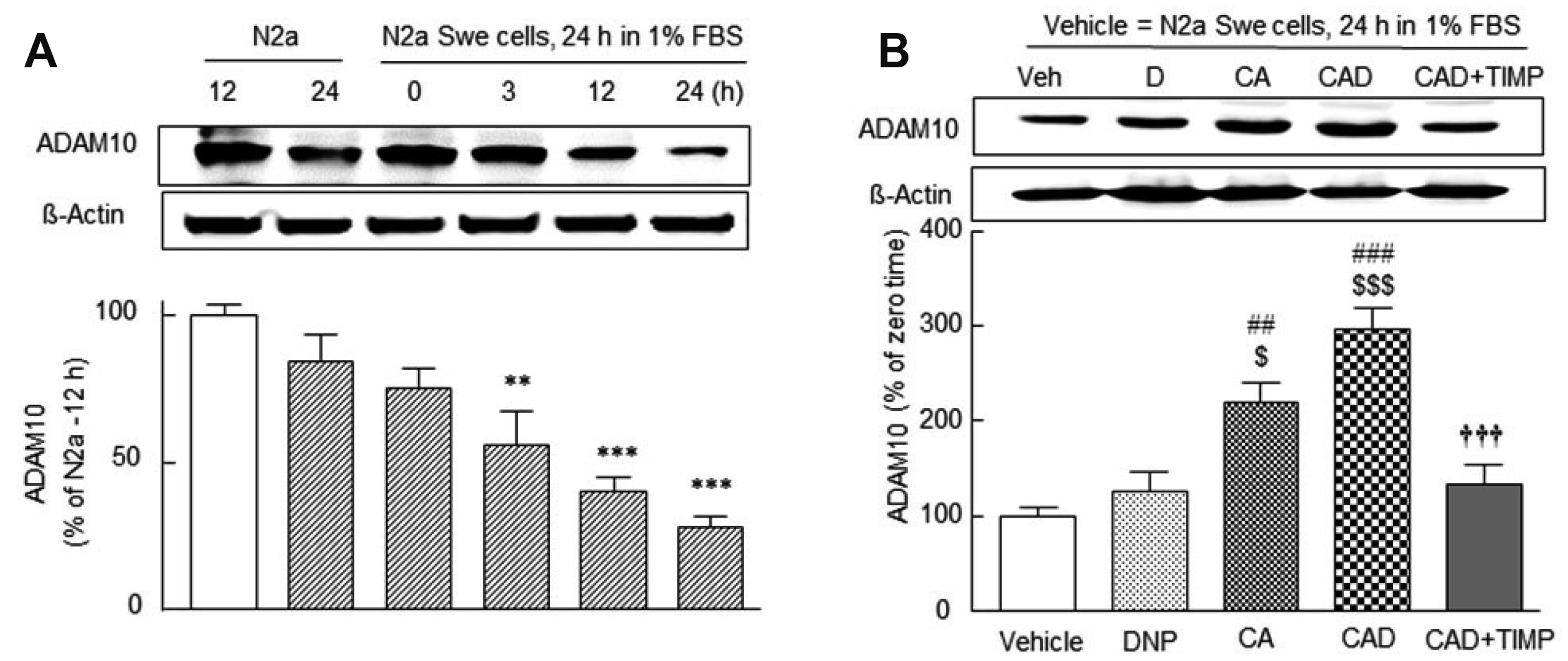
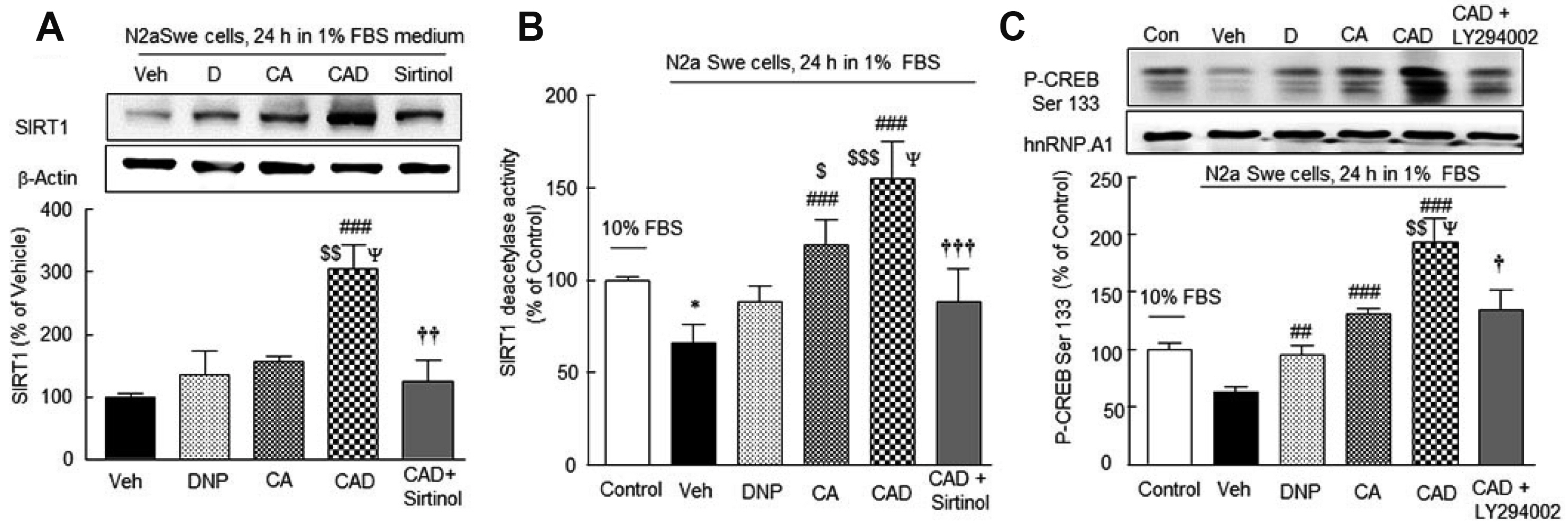
- Alzheimer’s disease (AD) is a multi-faceted neurodegenerative disease. Thus, current therapeutic strategies require multitarget-drug combinations to treat or prevent the disease. At the present time, single drugs have proven to be inadequate in terms of addressing the multifactorial pathology of AD, and multitarget-directed drug design has not been successful. Based on these points of views, it is judged that combinatorial drug therapies that target several pathogenic factors may offer more attractive therapeutic options. Thus, we explored that the combination therapy with lower doses of cilostazol and aripiprazole with add-on donepezil (CAD) might have potential in the pathogenesis of AD. In the present study, we found the superior efficacies of donepezil add-on with combinatorial mixture of cilostazol plus aripiprazole in modulation of expression of AD-relevant genes: Aβ accumulation, GSK-3β, P300, acetylated tau, phosphorylated-tau levels, and activation of α-secretase/ADAM 10 through SIRT1 activation in the N2a Swe cells expressing human APP Swedish mutation (N2a Swe cells). We also assessed that CAD synergistically raised acetylcholine release and choline acetyltransferase (CHAT) expression that were declined by increased β-amyloid level in the activated N2a Swe cells. Consequently, CAD treatment synergistically increased neurite elongation and improved cell viability through activations of PI3K, BDNF, β-catenin and a7-nicotinic cholinergic receptors in neuronal cells in the presence of Aβ1-42. This work endorses the possibility for efficient treatment of AD by supporting the synergistic therapeutic potential of donepezil add-on therapy in combination with lower doses of cilostazol and aripiprazole.
Keyword
Figure
Cited by 1 articles
-
The impact of CYP2D6 on donepezil concentration and its lack of effect on the treatment response and adverse effect in Korean patients with Alzheimer’s disease
Tae-Eun Kim, Jung-Woo Bae, Seongkuk Hong, Hong Jun Jeon, Yeonsil Moon
Korean J Physiol Pharmacol. 2025;29(2):227-233. doi: 10.4196/kjpp.24.239.
Reference
-
1. Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. 1999; Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 155:853–862. DOI: 10.1016/S0002-9440(10)65184-X. PMID: 10487842. PMCID: PMC1866907.2. Nunan J, Small DH. 2000; Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 483:6–10. DOI: 10.1016/S0014-5793(00)02076-7. PMID: 11033346.3. Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, Prinzen C, Endres K, Hiemke C, Blessing M, Flamez P, Dequenne A, Godaux E, van Leuven F, Fahrenholz F. 2004; A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 113:1456–1464. DOI: 10.1172/JCI20864. PMID: 15146243. PMCID: PMC406531.
Article4. Kojro E, Fahrenholz F. 2005; The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell Biochem. 38:105–127. DOI: 10.1007/0-387-23226-5_5. PMID: 15709475.5. Rojo AI, Sagarra MR, Cuadrado A. 2008; GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: relevance to exposure of neuronal cells to oxidative stress. J Neurochem. 105:192–202. DOI: 10.1111/j.1471-4159.2007.05124.x. PMID: 18005231.6. Ballatore C, Lee VM, Trojanowski JQ. 2007; Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 8:663–672. DOI: 10.1038/nrn2194. PMID: 17684513.
Article7. Hooper C, Killick R, Lovestone S. 2008; The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 104:1433–1439. DOI: 10.1111/j.1471-4159.2007.05194.x. PMID: 18088381. PMCID: PMC3073119.
Article8. Jämsä A, Hasslund K, Cowburn RF, Bäckström A, Vasänge M. 2004; The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer's disease-like tau phosphorylation. Biochem Biophys Res Commun. 319:993–1000. DOI: 10.1016/j.bbrc.2004.05.075. PMID: 15184080.
Article9. Lee HR, Park SY, Kim HY, Shin HK, Lee WS, Rhim BY, Hong KW, Kim CD. 2012; Protection by cilostazol against amyloid-β1-40-induced suppression of viability and neurite elongation through activation of CK2α in HT22 mouse hippocampal cells. J Neurosci Res. 90:1566–1576. DOI: 10.1002/jnr.23037. PMID: 22422579.
Article10. Counts SE, Mufson EJ. 2005; The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 64:263–272. DOI: 10.1093/jnen/64.4.263. PMID: 15835262.
Article11. Niewiadomska G, Baksalerska-Pazera M, Riedel G. 2009; The septo-hippocampal system, learning and recovery of function. Prog Neuropsychopharmacol Biol Psychiatry. 33:791–805. DOI: 10.1016/j.pnpbp.2009.03.039. PMID: 19389457.
Article12. Francis PT, Palmer AM, Snape M, Wilcock GK. 1999; The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 66:137–147. DOI: 10.1136/jnnp.66.2.137. PMID: 10071091. PMCID: PMC1736202.
Article13. Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. 2017; Molecular pathogenesis of Alzheimer's disease: an update. Ann Neurosci. 24:46–54. DOI: 10.1159/000464422. PMID: 28588356. PMCID: PMC5448443.
Article14. Anekonda TS, Reddy PH. 2006; Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem. 96:305–313. DOI: 10.1111/j.1471-4159.2005.03492.x. PMID: 16219030.
Article15. Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. 2006; Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 281:21745–21754. DOI: 10.1074/jbc.M602909200. PMID: 16751189.
Article16. Michán S, Li Y, Chou MM, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LE, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD. 2010; SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 30:9695–9707. DOI: 10.1523/JNEUROSCI.0027-10.2010. PMID: 20660252. PMCID: PMC2921958.17. Julien C, Tremblay C, Emond V, Lebbadi M, Salem N Jr, Bennett DA, Calon F. 2009; Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 68:48–58. DOI: 10.1097/NEN.0b013e3181922348. PMID: 19104446. PMCID: PMC2813570.
Article18. Tippmann F, Hundt J, Schneider A, Endres K, Fahrenholz F. 2009; Up-regulation of the alpha-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 23:1643–1654. DOI: 10.1096/fj.08-121392. PMID: 19144697.19. Lee HR, Shin HK, Park SY, Kim HY, Lee WS, Rhim BY, Hong KW, Kim CD. 2014; Cilostazol suppresses β-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-β. J Neurosci Res. 92:1581–1590. DOI: 10.1002/jnr.23421. PMID: 24903973.
Article20. Lee JH, Kim KY, Lee YK, Park SY, Kim CD, Lee WS, Rhim BY, Hong KW. 2004; Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther. 308:896–903. DOI: 10.1124/jpet.103.061853. PMID: 14634032.
Article21. Hong KW, Lee JH, Kima KY, Park SY, Lee WS. 2006; Cilostazol: therapeutic potential against focal cerebral ischemic damage. Curr Pharm Des. 12:565–573. DOI: 10.2174/138161206775474323. PMID: 16472148.
Article22. Park SH, Kim JH, Bae SS, Hong KW, Lee DS, Leem JY, Choi BT, Shin HK. 2011; Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid β-induced cognitive deficits associated with decreased amyloid β accumulation. Biochem Biophys Res Commun. 408:602–608. DOI: 10.1016/j.bbrc.2011.04.068. PMID: 21530492.
Article23. Burris KD, Molski TF, Xu C, Ryan E, Tottori K, Kikuchi T, Yocca FD, Molinoff PB. 2002; Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 302:381–389. DOI: 10.1124/jpet.102.033175. PMID: 12065741.
Article24. Croxtall JD. 2012; Aripiprazole: a review of its use in the management of schizophrenia in adults. CNS Drugs. 26:155–183. DOI: 10.2165/11208400-000000000-00000. PMID: 22296317.25. Pae CU, Forbes A, Patkar AA. 2011; Aripiprazole as adjunctive therapy for patients with major depressive disorder: overview and implications of clinical trial data. CNS Drugs. 25:109–127. DOI: 10.2165/11538980-000000000-00000. PMID: 21254788.26. Paulsen JS, Salmon DP, Thal LJ, Romero R, Weisstein-Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV. 2000; Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 54:1965–1971. DOI: 10.1212/WNL.54.10.1965. PMID: 10822438.
Article27. Eustace A, Coen R, Walsh C, Cunningham CJ, Walsh JB, Coakley D, Lawlor BA. 2002; A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer's disease. Int J Geriatr Psychiatry. 17:968–973. DOI: 10.1002/gps.736. PMID: 12325059.
Article28. De Deyn P, Jeste DV, Swanink R, Kostic D, Breder C, Carson WH, Iwamoto T. 2005; Aripiprazole for the treatment of psychosis in patients with Alzheimer's disease: a randomized, placebo-controlled study. J Clin Psychopharmacol. 25:463–467. DOI: 10.1097/01.jcp.0000178415.22309.8f. PMID: 16160622.29. Park SY, Shin HK, Lee WS, Bae SS, Kim K, Hong KW, Kim CD. 2017; Neuroprotection by aripiprazole against β-amyloid-induced toxicity by P-CK2α activation via inhibition of GSK-3β. Oncotarget. 8:110380–110391. DOI: 10.18632/oncotarget.22777. PMID: 29299155. PMCID: PMC5746390.
Article30. Guzior N, Wieckowska A, Panek D, Malawska B. 2015; Recent development of multifunctional agents as potential drug candidates for the treatment of Alzheimer's disease. Curr Med Chem. 22:373–404. DOI: 10.2174/0929867321666141106122628. PMID: 25386820. PMCID: PMC4435057.
Article31. Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. 1982; Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 215:1237–1239. DOI: 10.1126/science.7058341. PMID: 7058341.
Article32. Takada-Takatori Y, Kume T, Izumi Y, Ohgi Y, Niidome T, Fujii T, Sugimoto H, Akaike A. 2009; Roles of nicotinic receptors in acetylcholinesterase inhibitor-induced neuroprotection and nicotinic receptor up-regulation. Biol Pharm Bull. 32:318–324. DOI: 10.1248/bpb.32.318. PMID: 19252271.
Article33. Sugimoto H, Yamanishi Y, Iimura Y, Kawakami Y. 2000; Donepezil hydrochloride (E2020) and other acetylcholinesterase inhibitors. Curr Med Chem. 7:303–339. DOI: 10.2174/0929867003375191. PMID: 10637367.
Article34. Giacobini E. 2000; Cholinesterase inhibitors stabilize Alzheimer's disease. Ann N Y Acad Sci. 920:321–327. DOI: 10.1111/j.1749-6632.2000.tb06942.x. PMID: 11193171.35. Lee HR, Shin HK, Park SY, Kim HY, Lee WS, Rhim BY, Hong KW, Kim CD. 2014; Attenuation of β-amyloid-induced tauopathy via activation of CK2α/SIRT1: targeting for cilostazol. J Neurosci Res. 92:206–217. DOI: 10.1002/jnr.23310. PMID: 24254769.
Article36. Leroy K, Yilmaz Z, Brion JP. 2007; Increased level of active GSK-3beta in Alzheimer's disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol. 33:43–55. DOI: 10.1111/j.1365-2990.2006.00795.x. PMID: 17239007.
Article37. Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, Sato K. 2003; Spherical aggregates of beta-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3beta. Proc Natl Acad Sci U S A. 100:6370–6375. DOI: 10.1073/pnas.1237107100. PMID: 12750461. PMCID: PMC164453.38. Bannister AJ, Kouzarides T. 1996; The CBP co-activator is a histone acetyltransferase. Nature. 384:641–643. DOI: 10.1038/384641a0. PMID: 8967953.
Article39. Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. 2010; Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 67:953–966. DOI: 10.1016/j.neuron.2010.08.044. PMID: 20869593. PMCID: PMC3035103.
Article40. Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knäuper V, Docherty AJ, Murphy G. 2000; The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 473:275–279. DOI: 10.1016/S0014-5793(00)01528-3. PMID: 10818225.
Article41. Araki T, Sasaki Y, Milbrandt J. 2004; Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 305:1010–1013. DOI: 10.1126/science.1098014. PMID: 15310905.
Article42. Fusco S, Ripoli C, Podda MV, Ranieri SC, Leone L, Toietta G, McBurney MW, Schütz G, Riccio A, Grassi C, Galeotti T, Pani G. 2012; A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci U S A. 109:621–626. DOI: 10.1073/pnas.1109237109. PMID: 22190495. PMCID: PMC3258594.
Article43. Auld DS, Kar S, Quirion R. 1998; Beta-amyloid peptides as direct cholinergic neuromodulators: a missing link? Trends Neurosci. 21:43–49. DOI: 10.1016/S0166-2236(97)01144-2. PMID: 9464686.44. Potter PE, Rauschkolb PK, Pandya Y, Sue LI, Sabbagh MN, Walker DG, Beach TG. 2011; Pre- and post-synaptic cortical cholinergic deficits are proportional to amyloid plaque presence and density at preclinical stages of Alzheimer's disease. Acta Neuropathol. 122:49–60. DOI: 10.1007/s00401-011-0831-1. PMID: 21533854. PMCID: PMC3362487.
Article45. Takada-Takatori Y, Kume T, Ohgi Y, Fujii T, Niidome T, Sugimoto H, Akaike A. 2008; Mechanisms of alpha7-nicotinic receptor up-regulation and sensitization to donepezil induced by chronic donepezil treatment. Eur J Pharmacol. 590:150–156. DOI: 10.1016/j.ejphar.2008.06.027. PMID: 18585378.46. Votin V, Nelson WJ, Barth AI. 2005; Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J Cell Sci. 118(Pt 24):5699–5708. DOI: 10.1242/jcs.02679. PMID: 16303851. PMCID: PMC3373789.47. Rössler OG, Giehl KM, Thiel G. 2004; Neuroprotection of immortalized hippocampal neurones by brain-derived neurotrophic factor and Raf-1 protein kinase: role of extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase. J Neurochem. 88:1240–1252. DOI: 10.1046/j.1471-4159.2003.02255.x. PMID: 15009680.
Article48. Tapley P, Lamballe F, Barbacid M. 1992; K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 7:371–381. PMID: 1312698.49. Torii K, Nishizawa K, Kawasaki A, Yamashita Y, Katada M, Ito M, Nishimoto I, Terashita K, Aiso S, Matsuoka M. 2008; Anti-apoptotic action of Wnt5a in dermal fibroblasts is mediated by the PKA signaling pathways. Cell Signal. 20:1256–1266. DOI: 10.1016/j.cellsig.2008.02.013. PMID: 18407462.
Article50. Takada-Takatori Y, Kume T, Ohgi Y, Fujii T, Niidome T, Sugimoto H, Akaike A. 2008; Mechanisms of alpha7-nicotinic receptor up-regulation and sensitization to donepezil induced by chronic donepezil treatment. Eur J Pharmacol. 590:150–156. DOI: 10.1016/j.ejphar.2008.06.027. PMID: 18585378.51. Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schütz G. 2002; Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 31:47–54. DOI: 10.1038/ng882. PMID: 11967539.
Article52. Noriega LG, Feige JN, Canto C, Yamamoto H, Yu J, Herman MA, Mataki C, Kahn BB, Auwerx J. 2011; CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 12:1069–1076. DOI: 10.1038/embor.2011.151. PMID: 21836635. PMCID: PMC3185337.
Article53. Kosasa T, Kuriya Y, Matsui K, Yamanishi Y. 1999; Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats. Eur J Pharmacol. 380:101–107. DOI: 10.1016/S0014-2999(99)00545-2. PMID: 10513568.54. Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, Maeda T, Akaike A. 1997; Nicotinic receptor stimulation protects neurons against beta-amyloid toxicity. Ann Neurol. 42:159–163. DOI: 10.1002/ana.410420205. PMID: 9266724.55. Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. 2005; Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry. 162:676–682. DOI: 10.1176/appi.ajp.162.4.676. PMID: 15800138.
Article56. Kimura M, Akasofu S, Ogura H, Sawada K. 2005; Protective effect of donepezil against Abeta(1-40) neurotoxicity in rat septal neurons. Brain Res. 1047:72–84. DOI: 10.1016/j.brainres.2005.04.014. PMID: 15893738.57. Zimmermann M, Gardoni F, Marcello E, Colciaghi F, Borroni B, Padovani A, Cattabeni F, Di Luca M. 2004; Acetylcholinesterase inhibitors increase ADAM10 activity by promoting its trafficking in neuroblastoma cell lines. J Neurochem. 90:1489–1499. DOI: 10.1111/j.1471-4159.2004.02680.x. PMID: 15341532.
Article58. Zhang SJ, Xu TT, Li L, Xu YM, Qu ZL, Wang XC, Huang SQ, Luo Y, Luo NC, Lu P, Shi YF, Yang X, Wang Q. 2017; Bushen-Yizhi formula ameliorates cognitive dysfunction through SIRT1/ER stress pathway in SAMP8 mice. Oncotarget. 8:49338–49350. DOI: 10.18632/oncotarget.17638. PMID: 28521305. PMCID: PMC5564772.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Worsening of Psychotic Symptoms in Patients with Chronic Schizophrenia after Switching to or Combining Aripiprazole and Aripiprazole Monthly (Abilify Maintena)
- Inhibition of Amyloid beta Peptide-induced Neuronal Cytotoxicity by EGCG
- Protective Effect of Ginsenoside Rb1 and Rg1 Against beta Amyloid ( 25-35 )-Induced Neurotoxicity on B103 cells
- Splitomicin, a SIRT1 Inhibitor, Enhances Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- HO-1 Induced by Cilostazol Protects Against TNF-alpha-associated Cytotoxicity via a PPAR-gamma-dependent Pathway in Human Endothelial Cells