Int J Stem Cells.
2019 Mar;12(1):21-30. 10.15283/ijsc18040.
Splitomicin, a SIRT1 Inhibitor, Enhances Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungbuk National University, Cheongju, Korea. yhl4177@cbnu.ac.kr
- 2Biotechnology Research Institute, Chungbuk National University, Cheongju, Korea.
- 3Department of Radiation Oncology, College of Medicine, Chungbuk National University, Cheongju, Korea.
- 4Department of Microbiology, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2447221
- DOI: http://doi.org/10.15283/ijsc18040
Abstract
- BACKGROUND AND OBJECTIVES
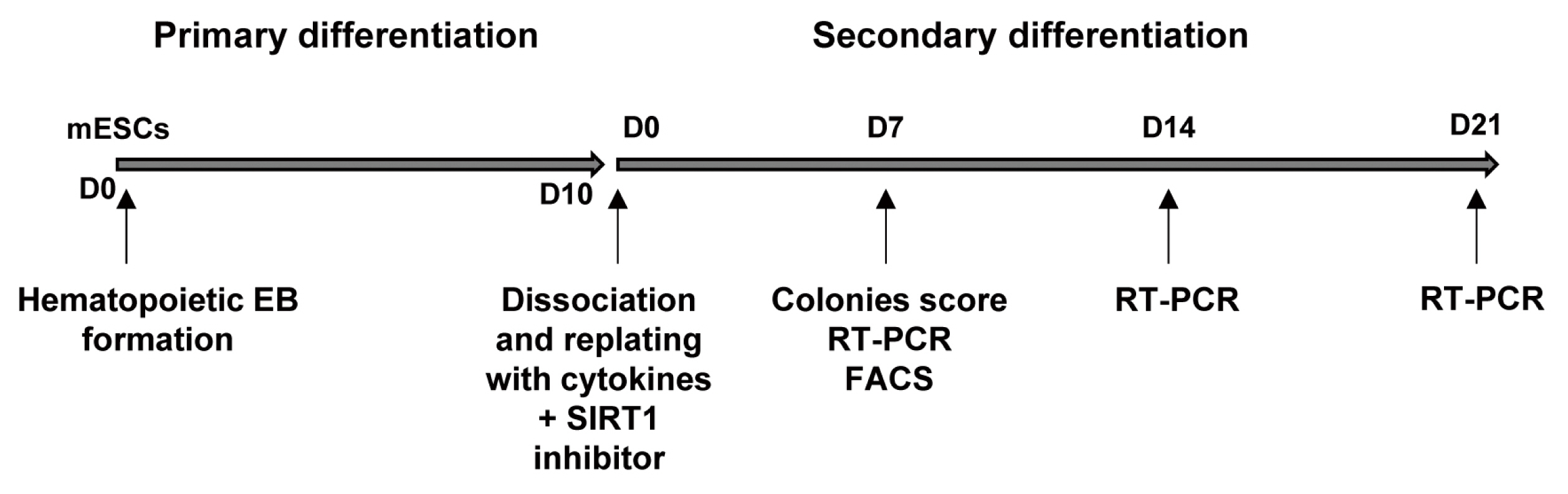
Embryonic stem (ES) cells have pluripotent ability to differentiate into multiple tissue lineages. SIRT1 is a class III histone deacetylase which modulates chromatin remodeling, gene silencing, cell survival, metabolism, and development. In this study, we examined the effects of SIRT1 inhibitors on the hematopoietic differentiation of mouse ES cells.
METHODS AND RESULTS
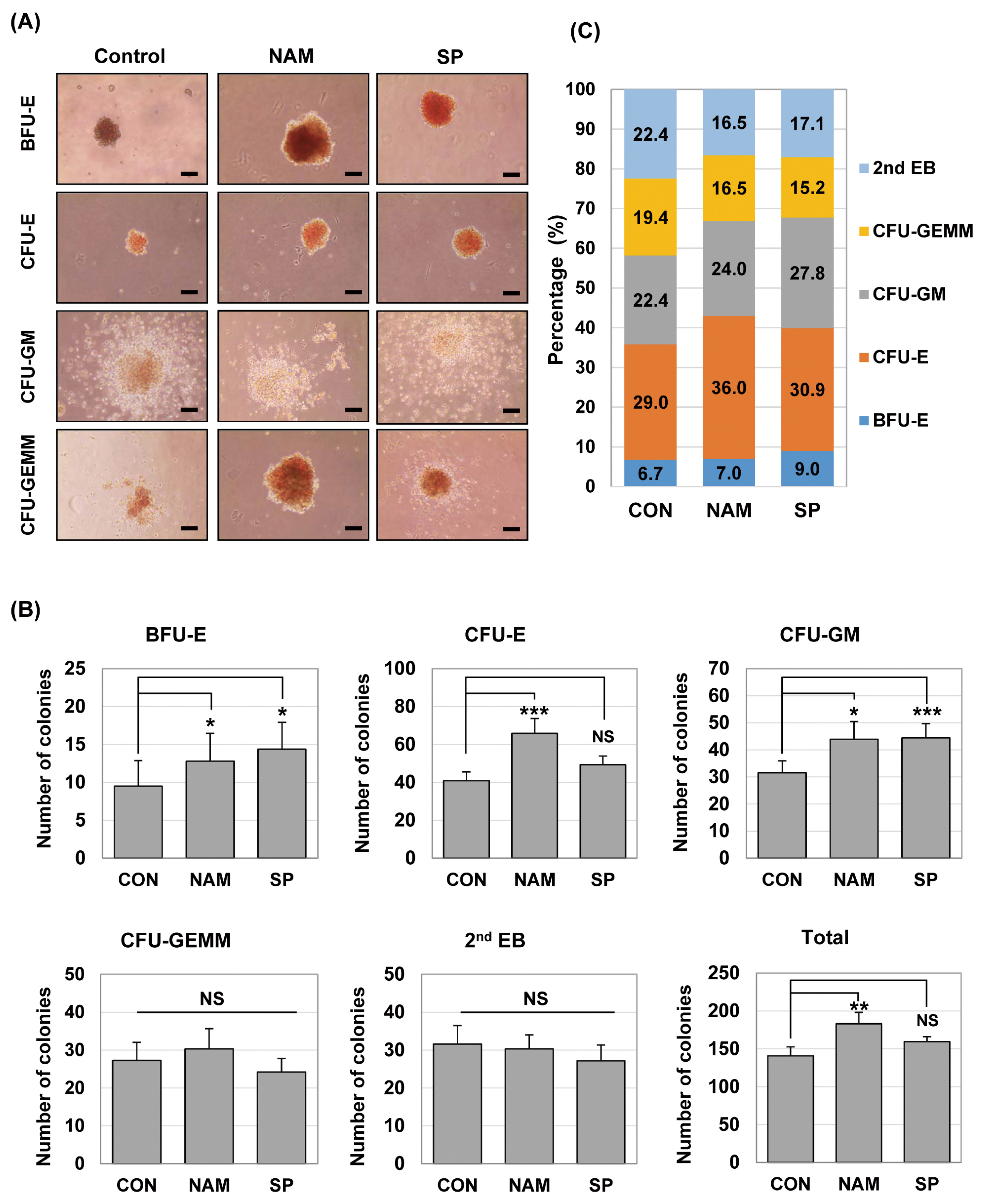
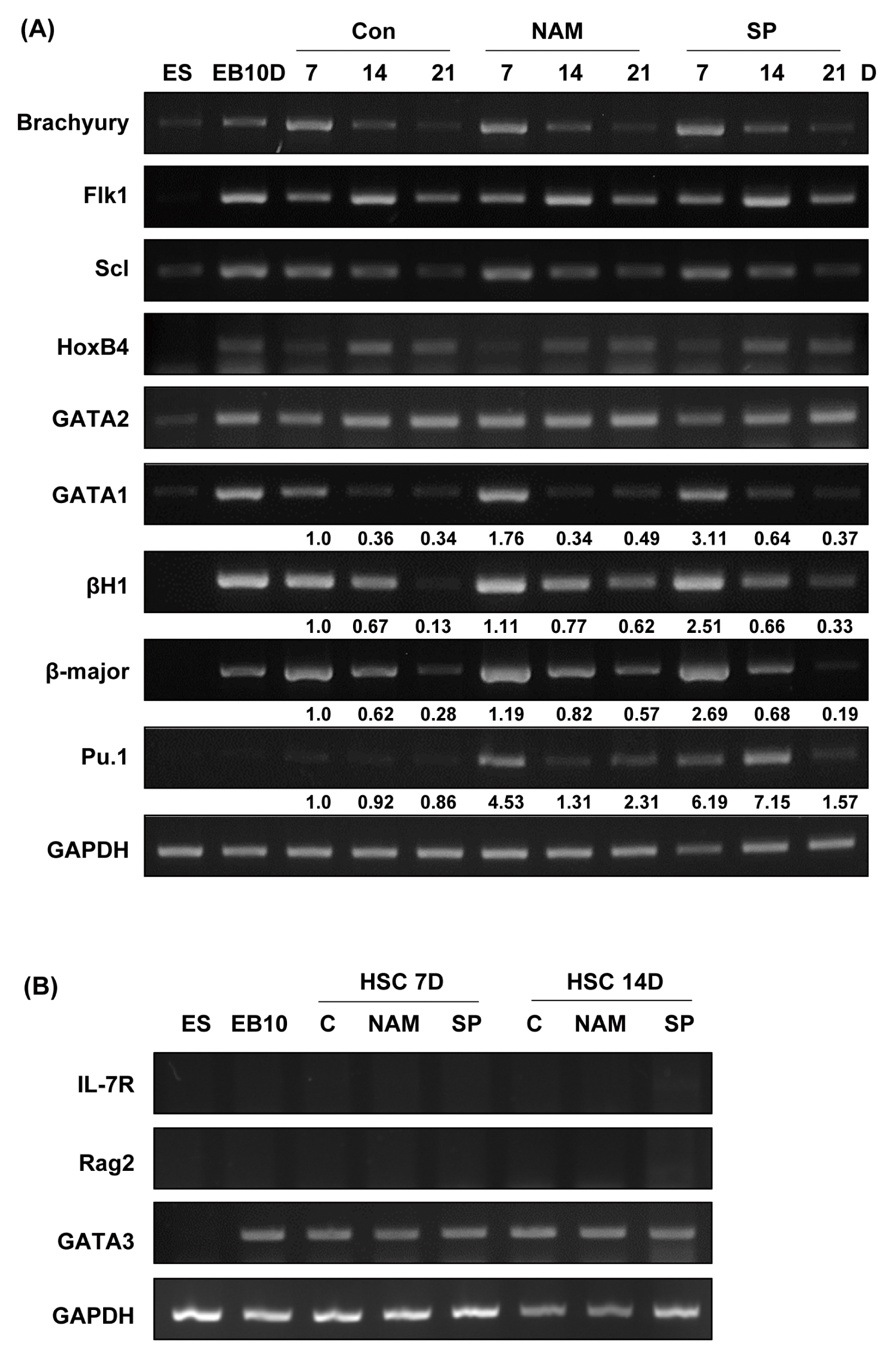
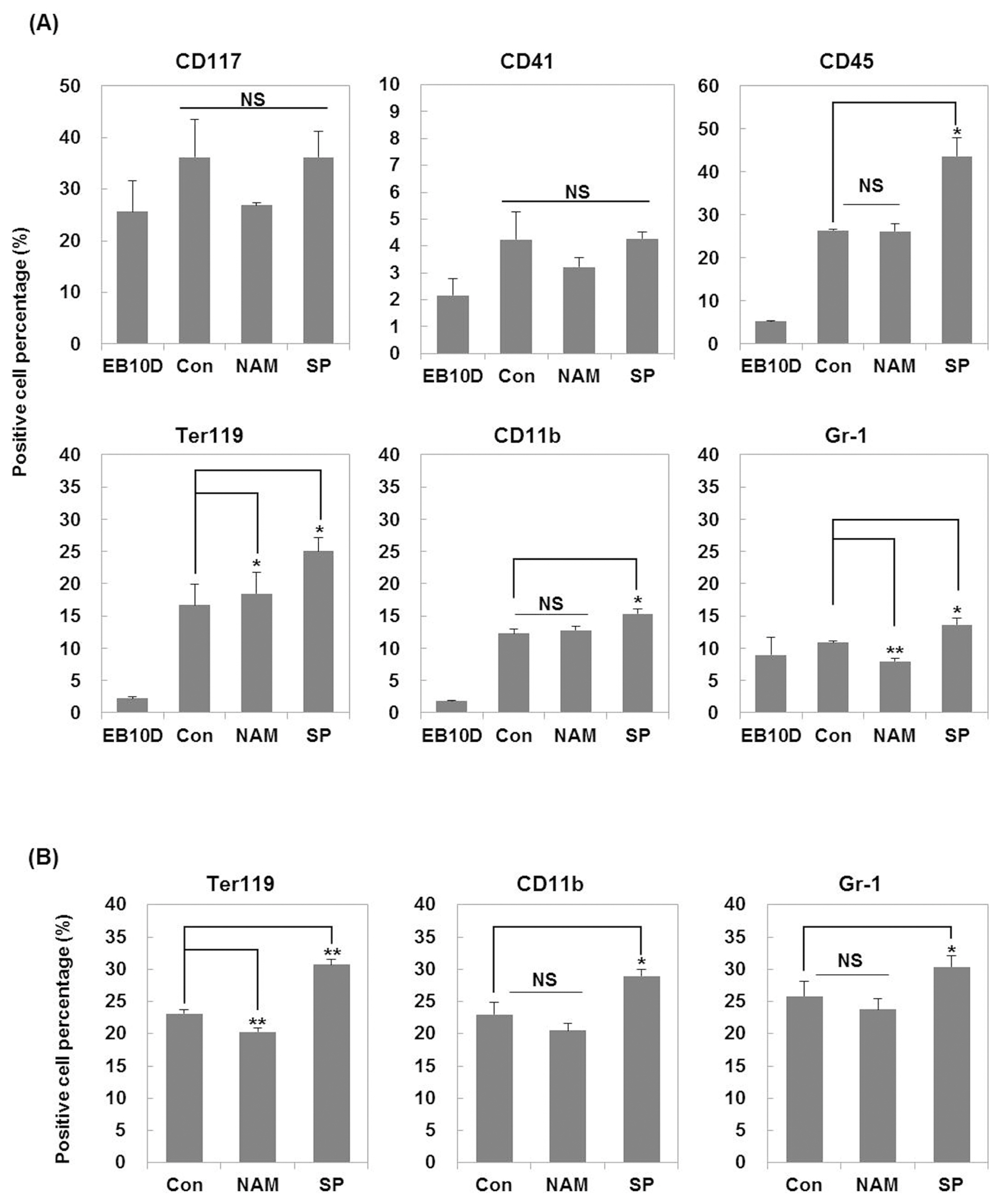
Treatment with the SIRT1 inhibitors, nicotinamide and splitomicin, during the hematopoietic differentiation of ES cells enhanced the production of hematopoietic progenitors and slightly up-regulated erythroid and myeloid specific gene expression. Furthermore, treatment with splitomicin increased the percentage of erythroid and myeloid lineage cells.
CONCLUSIONS
Application of the SIRT1 inhibitor splitomicin during ES cell differentiation to hematopoietic cells enhanced the yield of specific hematopoietic lineage cells from ES cells. This result suggests that SIRT1 is involved in the regulation of hematopoietic differentiation of specific lineages and that the modulation of the SIRT1 activity can be a strategy to enhance the efficiency of hematopoietic differentiation.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.
Article2. Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005; 6:872–884. DOI: 10.1038/nrm1744. PMID: 16227977.
Article3. Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009; 114:3513–3523. DOI: 10.1182/blood-2009-03-191304. PMID: 19652198. PMCID: 2766673.
Article4. Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995; 7:862–869. DOI: 10.1016/0955-0674(95)80071-9. PMID: 8608017.
Article5. Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993; 13:473–486. DOI: 10.1128/MCB.13.1.473. PMID: 8417345. PMCID: 358927.
Article6. Yamane T, Hosen N, Yamazaki H, Weissman IL. Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc Natl Acad Sci U S A. 2009; 106:8953–8958. DOI: 10.1073/pnas.0904090106. PMID: 19458045. PMCID: 2690003.
Article7. Wiles MV, Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991; 111:259–267. PMID: 1893864.
Article8. Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003; 102:906–915. DOI: 10.1182/blood-2003-03-0832. PMID: 12702499.
Article9. Herrmann BG. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991; 113:913–917. PMID: 1821859.
Article10. Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995; 376:62–66. DOI: 10.1038/376062a0. PMID: 7596435.
Article11. Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996; 86:47–57. DOI: 10.1016/S0092-8674(00)80076-8. PMID: 8689686.
Article12. Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992; 80:575–581. PMID: 1638017.
Article13. Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998; 12:2403–2412. DOI: 10.1101/gad.12.15.2403. PMID: 9694804. PMCID: 317050.
Article14. Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007; 404:1–13. DOI: 10.1042/BJ20070140. PMID: 17447894. PMCID: 2753453.
Article15. Sakamoto J, Miura T, Shimamoto K, Horio Y. Predominant expression of Sir2alpha, an NAD-dependent histone deacetylase, in the embryonic mouse heart and brain. FEBS Lett. 2004; 556:281–286. DOI: 10.1016/S0014-5793(03)01444-3. PMID: 14706864.
Article16. Homma K, Sone M, Taura D, Yamahara K, Suzuki Y, Takahashi K, Sonoyama T, Inuzuka M, Fukunaga Y, Tamura N, Itoh H, Yamanaka S, Nakao K. Sirt1 plays an important role in mediating greater functionality of human ES/iPS-derived vascular endothelial cells. Atherosclerosis. 2010; 212:42–47. DOI: 10.1016/j.atherosclerosis.2010.04.021. PMID: 20488443.
Article17. Ou X, Chae HD, Wang RH, Shelley WC, Cooper S, Taylor T, Kim YJ, Deng CX, Yoder MC, Broxmeyer HE. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011; 117:440–450. DOI: 10.1182/blood-2010-03-273011. PMID: 20966168. PMCID: 3031475.
Article18. Berger F, Ramírez-Hernández MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci. 2004; 29:111–118. DOI: 10.1016/j.tibs.2004.01.007. PMID: 15003268.
Article19. Denu JM. Vitamin B3 and sirtuin function. Trends Biochem Sci. 2005; 30:479–483. DOI: 10.1016/j.tibs.2005.07.004. PMID: 16039130.
Article20. Giammona LM, Fuhrken PG, Papoutsakis ET, Miller WM. Nicotinamide (vitamin B3) increases the polyploidisation and proplatelet formation of cultured primary human megakaryocytes. Br J Haematol. 2006; 135:554–566. DOI: 10.1111/j.1365-2141.2006.06341.x. PMID: 17054670.
Article21. Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer GN, Lerrer B, Cohen HY, Nagler A, Fibach E, Peled A. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012; 40:342–355.e1. DOI: 10.1016/j.exphem.2011.12.005. PMID: 22198152.22. Posakony J, Hirao M, Stevens S, Simon JA, Bedalov A. Inhibitors of Sir2: evaluation of splitomicin analogues. J Med Chem. 2004; 47:2635–2644. DOI: 10.1021/jm030473r. PMID: 15115404.
Article23. Neugebauer RC, Uchiechowska U, Meier R, Hruby H, Valkov V, Verdin E, Sippl W, Jung M. Structure-activity studies on splitomicin derivatives as sirtuin inhibitors and computational prediction of binding mode. J Med Chem. 2008; 51:1203–1213. DOI: 10.1021/jm700972e. PMID: 18269226.
Article24. Liu FC, Liao CH, Chang YW, Liou JT, Day YJ. Splitomicin suppresses human platelet aggregation via inhibition of cyclic AMP phosphodiesterase and intracellular Ca++ release. Thromb Res. 2009; 124:199–207. DOI: 10.1016/j.thromres.2009.02.013. PMID: 19327818.
Article25. Park JA, Kim YE, Seok HJ, Park WY, Kwon HJ, Lee Y. Differentiation and upregulation of heat shock protein 70 induced by a subset of histone deacetylase inhibitors in mouse and human embryonic stem cells. BMB Rep. 2011; 44:176–181. DOI: 10.5483/BMBRep.2011.44.3.176. PMID: 21429295.
Article26. Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H, Nishikawa S. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991; 174:63–71. DOI: 10.1084/jem.174.1.63. PMID: 1711568. PMCID: 2118893.
Article27. Robin C, Ottersbach K, Boisset JC, Oziemlak A, Dzierzak E. CD41 is developmentally regulated and differentially expressed on mouse hematopoietic stem cells. Blood. 2011; 117:5088–5091. DOI: 10.1182/blood-2011-01-329516. PMID: 21415271. PMCID: 3109535.
Article28. McKinney-Freeman SL, Naveiras O, Yates F, Loewer S, Philitas M, Curran M, Park PJ, Daley GQ. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009; 114:268–278. DOI: 10.1182/blood-2008-12-193888. PMID: 19420357. PMCID: 2714203.
Article29. Kina T, Ikuta K, Takayama E, Wada K, Majumdar AS, Weissman IL, Katsura Y. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000; 109:280–287. DOI: 10.1046/j.1365-2141.2000.02037.x. PMID: 10848813.
Article30. Lai L, Alaverdi N, Maltais L, Morse HC 3rd. Mouse cell surface antigens: nomenclature and immunophenotyping. J Immunol. 1998; 160:3861–3868. PMID: 9558091.31. Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993; 151:2399–2408. PMID: 8360469.32. Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat Rev Mol Cell Biol. 2016; 17:170–182. DOI: 10.1038/nrm.2015.27. PMID: 26818440.
Article33. Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002; 109:29–37. DOI: 10.1016/S0092-8674(02)00680-3. PMID: 11955444.
Article34. Jackson M, Ma R, Taylor AH, Axton RA, Easterbrook J, Kydonaki M, Olivier E, Marenah L, Stanley EG, Elefanty AG, Mountford JC, Forrester LM. Enforced expression of HOXB4 in human embryonic stem cells enhances the production of hematopoietic progenitors but has no effect on the maturation of red blood cells. Stem Cells Transl Med. 2016; 5:981–990. DOI: 10.5966/sctm.2015-0324. PMID: 27352929. PMCID: 4954454.
Article35. Ding X, Lin Q, Ensenat-Waser R, Rose-John S, Zenke M. Polycomb group protein Bmi1 promotes hematopoietic cell development from embryonic stem cells. Stem Cells Dev. 2012; 21:121–132. DOI: 10.1089/scd.2010.0539. PMID: 21545235.
Article36. Han MK, Song EK, Guo Y, Ou X, Mantel C, Broxmeyer HE. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008; 2:241–251. DOI: 10.1016/j.stem.2008.01.002. PMID: 18371449. PMCID: 2819008.
Article37. Calvanese V, Lara E, Suárez-Alvarez B, Abu Dawud R, Vázquez-Chantada M, Martínez-Chantar ML, Embade N, López-Nieva P, Horrillo A, Hmadcha A, Soria B, Piazzolla D, Herranz D, Serrano M, Mato JM, Andrews PW, López-Larrea C, Esteller M, Fraga MF. Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc Natl Acad Sci U S A. 2010; 107:13736–13741. DOI: 10.1073/pnas.1001399107. PMID: 20631301. PMCID: 2922228.
Article38. Saunders LR, Sharma AD, Tawney J, Nakagawa M, Okita K, Yamanaka S, Willenbring H, Verdin E. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY). 2010; 2:415–431. DOI: 10.18632/aging.100176. PMID: 20634564. PMCID: 2933889.
Article39. Matsui K, Ezoe S, Oritani K, Shibata M, Tokunaga M, Fujita N, Tanimura A, Sudo T, Tanaka H, McBurney MW, Matsumura I, Kanakura Y. NAD-dependent histone deacetylase, SIRT1, plays essential roles in the maintenance of hematopoietic stem cells. Biochem Biophys Res Commun. 2012; 418:811–817. DOI: 10.1016/j.bbrc.2012.01.109. PMID: 22306819.
Article40. Leko V, Varnum-Finney B, Li H, Gu Y, Flowers D, Nourigat C, Bernstein ID, Bedalov A. SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood. 2012; 119:1856–1860. DOI: 10.1182/blood-2011-09-377077. PMID: 22219225. PMCID: 3293640.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- SIRT1 Inhibits p53 but not NF-kappaB Transcriptional Activity during Differentiation of Mouse Embryonic Stem Cells into Embryoid Bodies
- Assessment of Developmental Toxicants using Human Embryonic Stem Cells
- Differentiation Culture of Hematopoietic Stem Cells from Embryonic Stem Cells
- Inhibition of MUC1-C Increases ROS and Cell Death in Mouse Embryonic Stem Cells
- Generation of hematopoietic stem cells from human embryonic stem cells using a defined, stepwise, serum-free, and serum replacement-free monolayer culture method