Korean J Physiol Pharmacol.
2011 Apr;15(2):83-88. 10.4196/kjpp.2011.15.2.83.
HO-1 Induced by Cilostazol Protects Against TNF-alpha-associated Cytotoxicity via a PPAR-gamma-dependent Pathway in Human Endothelial Cells
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, and MRC for Ischemic Tissue Regeneration and Medical Research Institute, Pusan National University, Yangsan 626-770, Korea. chidkim@pusan.ac.kr
- KMID: 2285411
- DOI: http://doi.org/10.4196/kjpp.2011.15.2.83
Abstract
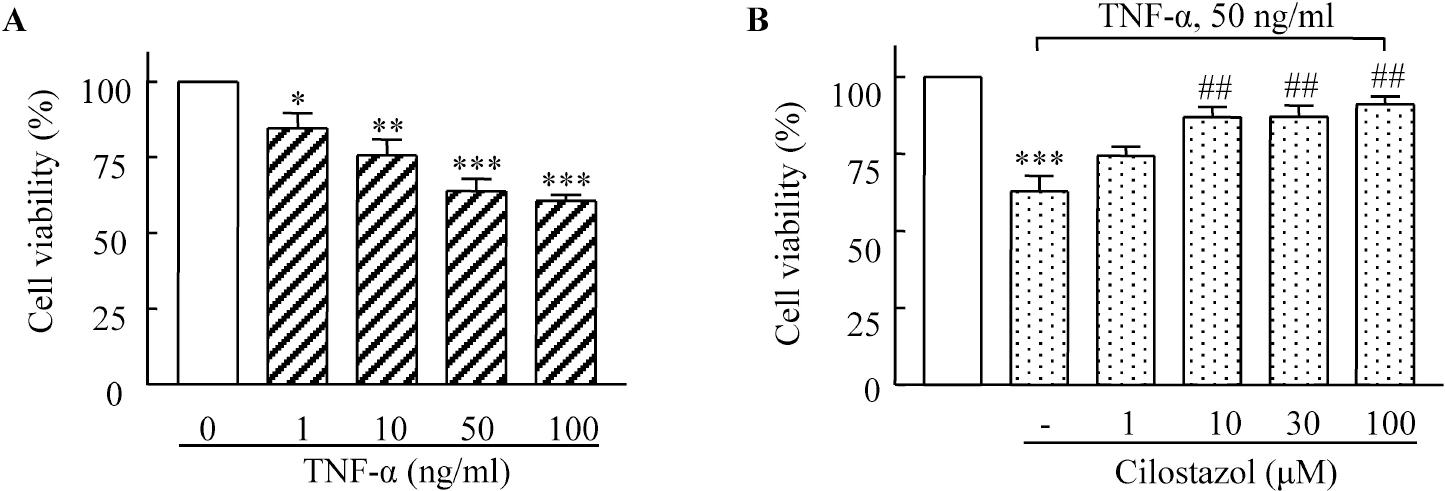
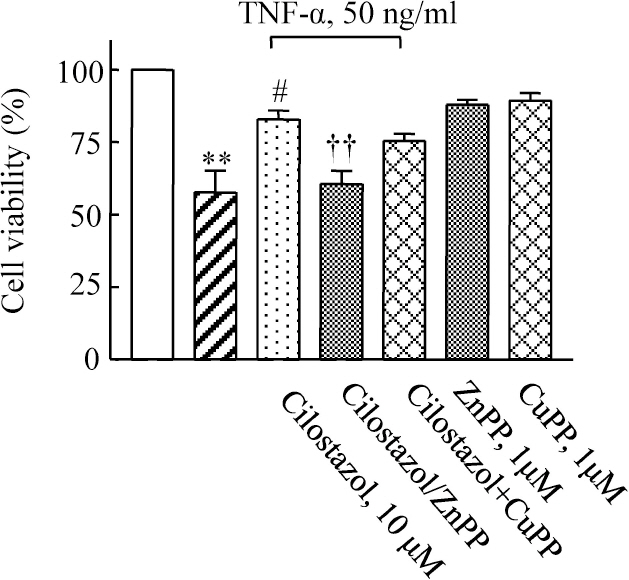
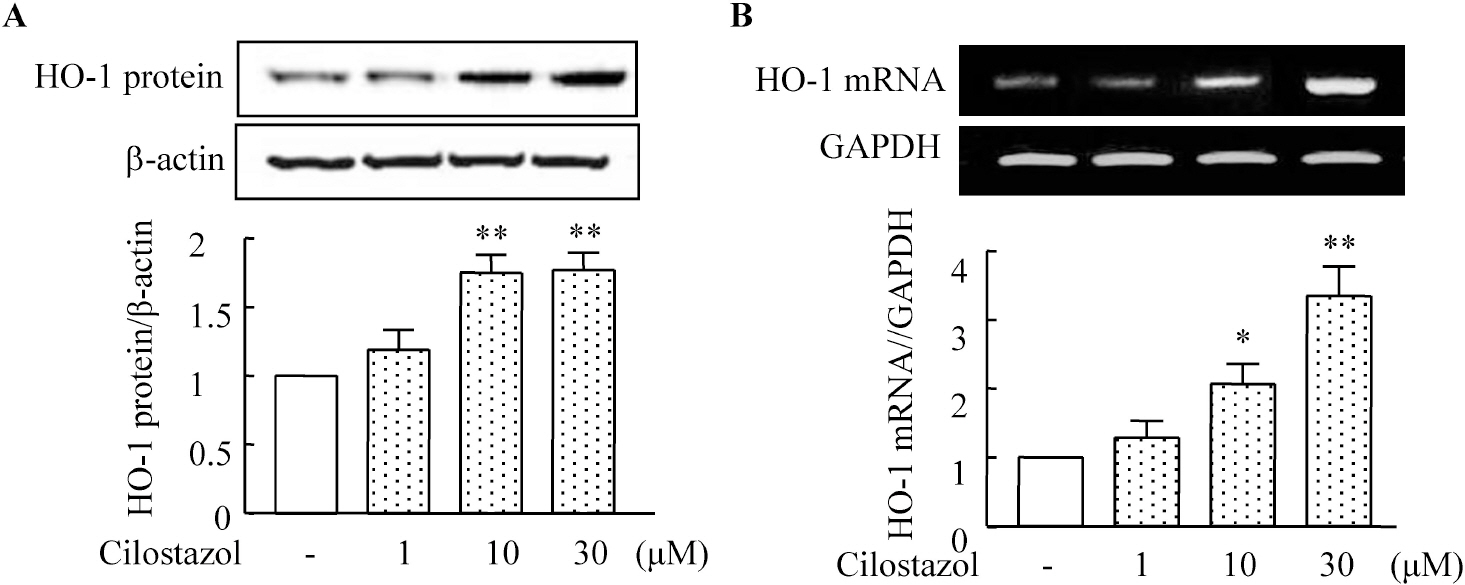
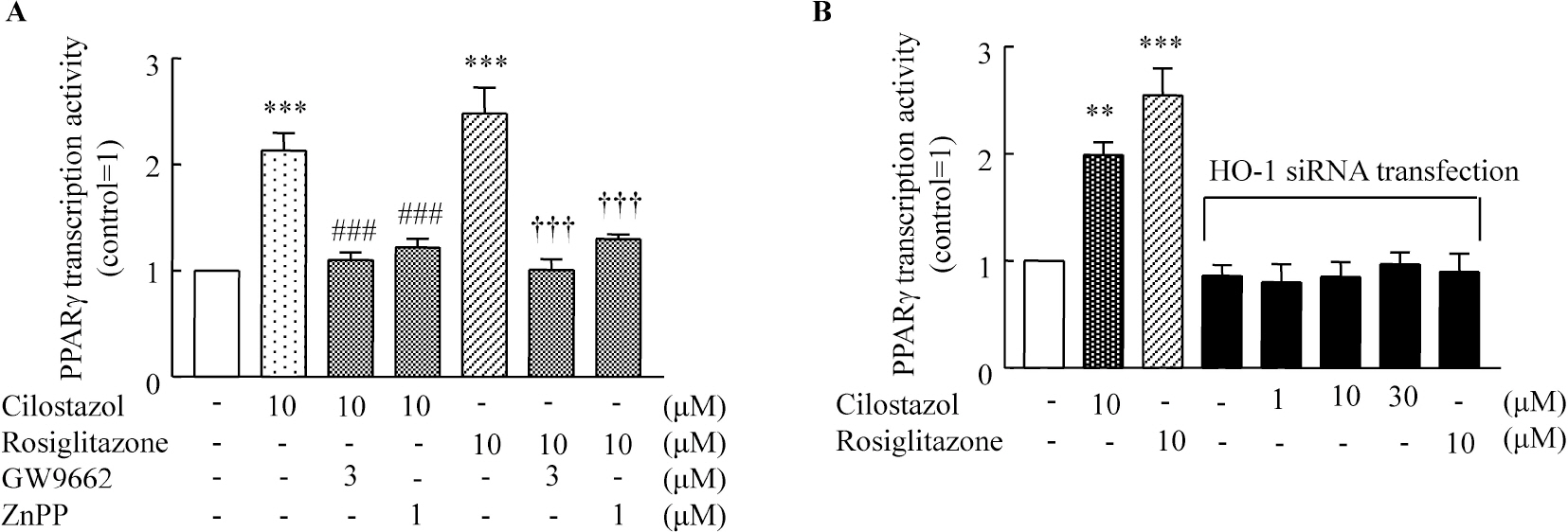
- A large body of evidence has indicated that induction of endogenous antioxidative proteins seems to be a reasonable strategy for delaying the progression of cell injury. In our previous study, cilostazol was found to increase the expression of the antioxidant enzyme heme oxygenase-1 (HO-1) in synovial cells. Thus, the present study was undertaken to examine whether cilostazol is able to counteract tumor necrosis factor-alpha (TNF-alpha)-induced cell death in endothelial cells via the induction of HO-1 expression. We exposed human umbilical vein endothelial cells (HUVECs) to TNF-alpha (50 ng/ml), with or without cilostazol (10 microM). Pretreatment with cilostazol markedly reduced TNF-alpha-induced viability loss in the HUVECs, which was reversed by zinc protoporphyrine IX (ZnPP), an inhibitor of HO-1. Moreover, cilostazol increased HO-1 protein and mRNA expression. Cilostazol-induced HO-1 induction was markedly attenuated not only by ZnPP but also by copper-protoporphyrin IX (CuPP). In an assay measuring peroxisome proliferator-activated receptor-gamma (PPAR-gamma) transcription activity, cilostazol directly increased PPAR-gamma transcriptional activity which was completely abolished by HO-1 inhibitor. Furthermore, increased PPAR-gamma activity by cilostazol and rosiglitazone was completely abolished in cells transfected with HO-1 siRNA. Taken together, these results indicate that cilostazol up-regulates HO-1 and protects cells against TNF-alpha-induced endothelial cytotoxicity via a PPAR-gamma-dependent pathway.
Keyword
MeSH Terms
-
Cell Death
Endothelial Cells
Heme Oxygenase-1
Human Umbilical Vein Endothelial Cells
Humans
Peroxisomes
Proteins
RNA, Messenger
RNA, Small Interfering
Tetrazoles
Thiazolidinediones
Tumor Necrosis Factor-alpha
Zinc
Heme Oxygenase-1
Proteins
RNA, Messenger
RNA, Small Interfering
Tetrazoles
Thiazolidinediones
Tumor Necrosis Factor-alpha
Zinc
Figure
Cited by 2 articles
-
Regulation of Ca2+ Signaling in Pulmonary Hypertension
Amy L. Firth, Jun Yeon Won, Won Sun Park
Korean J Physiol Pharmacol. 2013;17(1):1-8. doi: 10.4196/kjpp.2013.17.1.1.Involvement of Heme Oxygenase-1 in Orexin-A-induced Angiogenesis in Vascular Endothelial Cells
Mi-Kyoung Kim, Hyun-Joo Park, Su-Ryun Kim, Yoon Kyung Choi, Soo-Kyung Bae, Moon-Kyoung Bae
Korean J Physiol Pharmacol. 2015;19(4):327-334. doi: 10.4196/kjpp.2015.19.4.327.
Reference
-
References
1. Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006; 126:789–799.2. Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999; 99:3125–3131.3. Duan SZ, Ivashchenko CY, Usher MG, Mortensen RM. PPAR-gamma in the Cardiovascular System. PPAR Res. 2008; 2008:745–804.4. Touyz RM, Schiffrin EL. Peroxisome proliferator-activated receptors in vascular biology-molecular mechanisms and clinical implications. Vascul Pharmacol. 2006; 45:19–28.
Article5. Nisbet RE, Sutliff RL, Hart CM. The role of peroxisome proliferator-activated receptors in pulmonary vascular disease. PPAR Res. 2007; 2007:18797.
Article6. Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPAR-gamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008; 118:1846–1857.7. Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The Role of PPARs in Lung Fibrosis. PPAR Res. 2007; 2007:71323.
Article8. Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol. 2006; 6:421–427.
Article9. Chen YE, Fu M, Zhang J, Zhu X, Lin Y, Akinbami MA, Song Q. Peroxisome proliferator-activated receptors and the cardiovascular system. Vitam Horm. 2003; 66:157–188.
Article10. Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, Libby P, Plutzky J, Luster AD. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000; 164:6503–6508.11. Kim KY, Shin HK, Choi JM, Hong KW. Inhibition of lipopolysaccharide-induced apoptosis by cilostazol in human umbilical vein endothelial cells. J Pharmacol Exp Ther. 2002; 300:709–715.
Article12. Hong KW, Kim KY, Shin HK, Lee JH, Choi JM, Kwak YG, Kim CD, Lee WS, Rhim BY. Cilostazol prevents tumor necrosis factor-alpha-induced cell death by suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation and activation of Akt/cyclic AMP response element-binding protein phosphorylation. J Pharmacol Exp Ther. 2003; 306:1182–1190.13. Choi JM, Shin HK, Kim KY, Lee JH, Hong KW. Neuroprotective effect of cilostazol against focal cerebral ischemia via antiapoptotic action in rats. J Pharmacol Exp Ther. 2002; 300:787–793.
Article14. Park SY, Lee SW, Shin HK, Chung WT, Lee WS, Rhim BY, Hong KW, Kim CD. Cilostazol enhances apoptosis of synovial cells from rheumatoid arthritis patients with inhibition of cytokine formation via Nrf2-linked heme oxygenase 1 induction. Arthritis Rheum. 2010; 62:732–741.
Article15. Zhou H, Liu H, Porvasnik SL, Terada N, Agarwal A, Cheng Y, Visner GA. Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Lab Invest. 2006; 86:62–71.
Article16. Idriss NK, Blann AD, Lip GY. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008; 52:971–978.
Article17. Stanford SJ, Walters MJ, Hislop AA, Haworth SG, Evans TW, Mann BE, Motterlini R, Mitchell JA. Heme oxygenase is expressed in human pulmonary artery smooth muscle where carbon monoxide has an anti-proliferative role. Eur J Pharmacol. 2003; 473:135–141.
Article18. Christou H, Morita T, Hsieh CM, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000; 86:1224–1229.
Article19. Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001; 98:8798–8803.
Article20. Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005; 25:1786–1795.
Article21. Hoekstra KA, Godin DV, Cheng KM. Protective role of heme oxygenase in the blood vessel wall during atherogenesis. Biochem Cell Biol. 2004; 82:351–359.
Article22. Risérus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L, Strum JC, Basu S, Cheeseman J, Fielding BA, Humphreys SM, Danoff T, Moore N R, Murgatroyd P, O'Rahilly S, Sutton P, Willson T, Hassall D, Frayn KN, Karpe F. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008; 57:332–339.23. Krönke G, Kadl A, Ikonomu E, Blüml S, Fürnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR, Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007; 27:1276–1282.24. Kintscher U, Lyon C, Wakino S, Bruemmer D, Feng X, Goetze S, Graf K, Moustakas A, Staels B, Fleck E, Hsueh WA, Law RE. PPARalpha inhibits TGF-beta-induced beta5 integrin transcription in vascular smooth muscle cells by interacting with Smad4. Circ Res. 2002; 91:e35–44.
Article25. Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008; 10:1767–1812.
Article26. Immenschuh S, Kietzmann T, Hinke V, Wiederhold M, Katz N, Muller-Eberhard U. The rat heme oxygenase-1 gene is transcriptionally induced via the protein kinase A signaling pathway in rat hepatocyte cultures. Mol Pharmacol. 1998; 53:483–491.
Article27. Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med. 2006; 41:1079–1091.
Article28. Lee JH, Kim KY, Lee YK, Park SY, Kim CD, Lee WS, Rhim BY, Hong KW. Cilostazol prevents focal cerebral ischemic injury by enhancing casein kinase 2 phosphorylation and suppression of phosphatase and tensin homolog deleted from chromosome 10 phosphorylation in rats. J Pharmacol Exp Ther. 2004; 308:896–903.
Article29. Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: prevention by cilostazol. Circulation. 2004; 109:1022–1028.30. Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, Ghoneim SM, de Lange WJ, Keen HL, Tsai YS, Maeda N, Sigmund CD, Faraci FM. Interference with PPAR-gamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008; 51:867–871.31. Fan CD, Zhao BX, Wei F, Zhang GH, Dong WL, Miao JY. Synthesis and discovery of autophagy inducers for A549 and H460 lung cancer cells, novel 1-(2′-hydroxy-3′-aroxypropyl)-3-aryl-1H-pyrazole-5-carbohydrazide derivatives. Bioorg Med Chem Lett. 2008; 18:3860–3864.
Article32. Lee CH, Chawla A, Urbiztondo N, Liao D, Boisvert WA, Evans RM, Curtiss LK. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003; 302:453–457.33. Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007; 115:518–533.
Article34. Li MY, Yuan H, Ma LT, Kong AW, Hsin MK, Yip JH, Underwood MJ, Chen GG. Roles of peroxisome proliferator-activated receptor-alpha and -gamma in the development of non-small cell lung cancer. Am J Respir Cell Mol Biol. 2010; 43:674–683.35. DiDonato M, Narindrasorasak S, Sarkar B. Expression, purification, and metal binding characteristics of the putative copper binding domain from the Wilson disease copper transporting ATPase (ATP7B). Adv Exp Med Biol. 1999; 448:165–173.
Article36. Merkel KD, Erdmann JM, McHugh KP, Abu-Amer Y, Ross FP, Teitelbaum SL. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999; 154:203–210.37. Libby P, Sukhova G, Lee RT, Galis ZS. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J Cardiovasc Pharmacol. 1995; 25 Suppl. 2:S9–12.
Article38. Wu JS, Lin TN, Wu KK. Rosiglitazone and PPAR-gamma overexpression protect mitochondrial membrane potential and prevent apoptosis by upregulating anti-apoptotic Bcl-2 family proteins. J Cell Physiol. 2009; 220:58–71.39. Mun L, Jun MS, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Son KH, Lee DH, Kim YS, Park K, Chang KC. 7,8-didehydro-cimigenol from Cimicifugae rhizoma inhibits TNF-α-induced VCAM-1 but not ICAM-1expression through upregulation of PPAR-γ in human endothelial cells. Food Chem Toxicol. 2011; 49:166–172.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genipin Selectively Inhibits TNF-alpha-activated VCAM-1 But Not ICAM-1 Expression by Upregulation of PPAR-gamma in Human Endothelial Cells
- Effects of Peroxisome Proliferator-Activated Receptor-gamma on the Production of Tumor Necrosis Factor-alpha in Stimulated Human Monocoytes
- Synergistic Efficacy of Concurrent Treatment with Cilostazol and Probucol on the Suppression of Reactive Oxygen Species and Inflammatory Markers in Cultured Human Coronary Artery Endothelial Cells
- Saccharomyces boulardii Activates Expression of Peroxisome Proliferator-activated Receptor-gamma in HT-29 Cells
- Effect of cytokines on the expression of cell adhesion molecule and on the adhesion of melanoma cells to endothelial cells