Cancer Res Treat.
2020 Jan;52(1):246-253. 10.4143/crt.2019.189.
Immunogenicity and Optimal Timing of 13-Valent Pneumococcal Conjugate Vaccination during Adjuvant Chemotherapy in Gastric and Colorectal Cancer: A Randomized Controlled Trial
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University College of Medicine, Hwasun, Korea
- 2Department of Hematology/Oncology, School of Medicine, Kyungpook National University, Daegu, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Yonsei University of College of Medicine, Seoul, Korea
- 4Department of Preventive Medicine, Chonnam National University Medical School, Hwasun, Korea
- 5Department of Biostatistics and Bioinformatics, Duke University, Durham, NC, USA
- 6Division of Infectious Disease, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- KMID: 2501216
- DOI: http://doi.org/10.4143/crt.2019.189
Abstract
- Purpose
Pneumococcal vaccination (13-valent pneumococcal conjugate vaccine [PCV13]) is recommended to cancer patients undergoing systemic chemotherapy. However, the optimal time interval between vaccine administration and initiation of chemotherapy has been little studied in adult patients with solid malignancies.
Materials and Methods
We conducted a prospective randomized controlled trial to evaluate whether administering PCV13 on the first day of chemotherapy is non-inferior to vaccinating 2 weeks prior to chemotherapy initiation. Patients were randomly assigned to two study arms, and serum samples were collected at baseline and 4 weeks after vaccination to analyze the serologic response against Streptococcus pneumoniae using a multiplexed opsonophagocytic killing assay.
Results
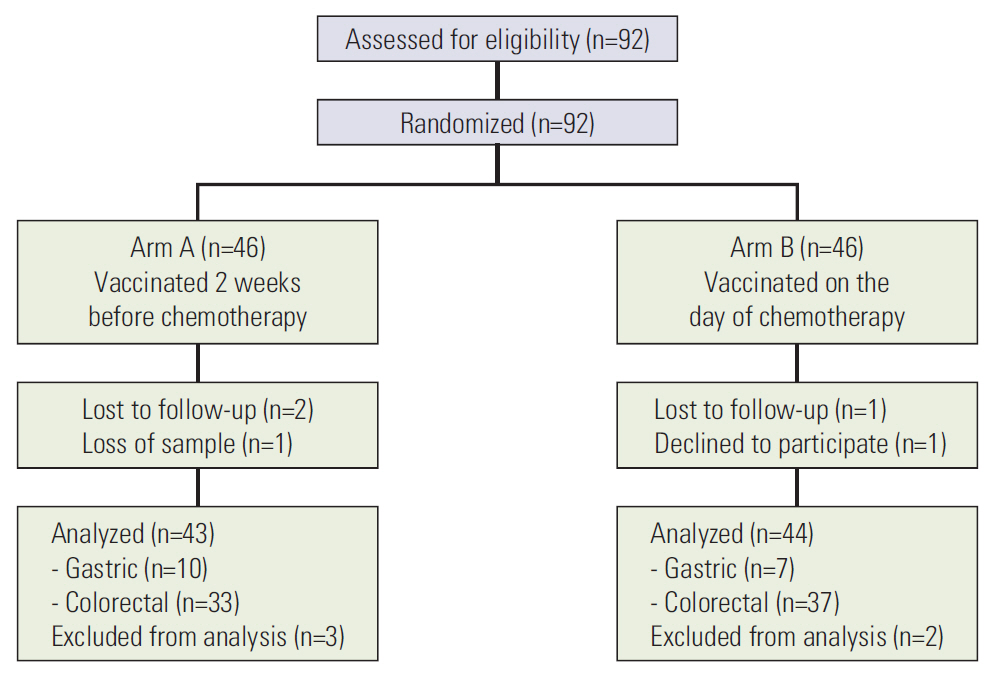
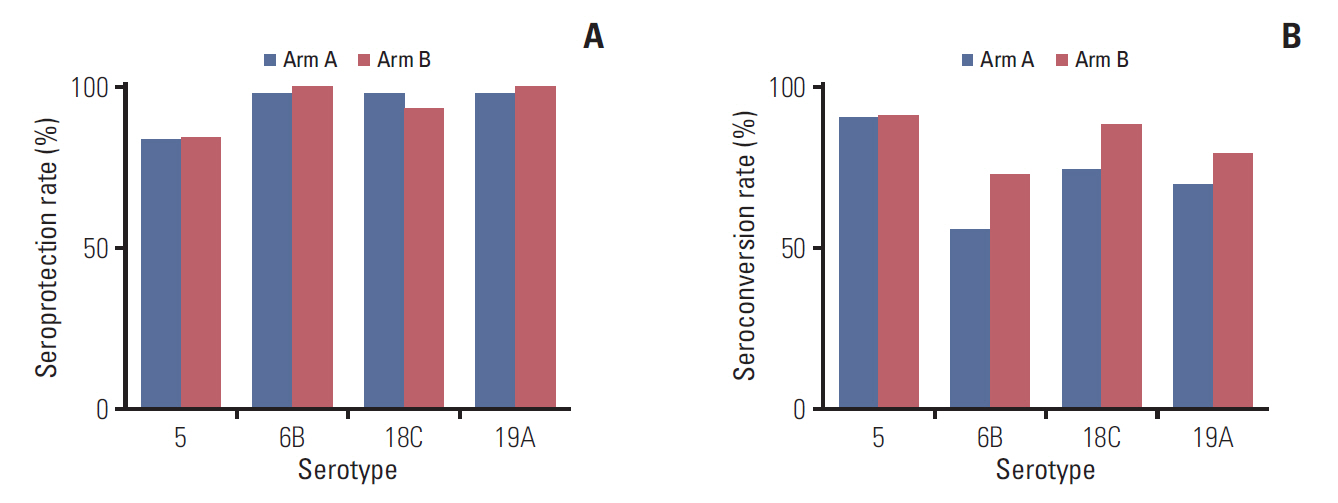
Of the 92 patients who underwent randomization, 43 patients in arm A (vaccination 2 weeks before chemotherapy) and 44 patients in arm B (vaccination on the first day of chemotherapy) were analyzed. Immunogenicity was assessed by geometric mean and fold-increase of post-vaccination titers, seroprotection rates (percentage of patients with post-vaccination titers > 1:64), and seroconversion rates (percentage of patients with > 4-fold increase in post-vaccination titers). Serologic responses to PCV13 did not differ significantly between the two study arms according to all three types of assessments.
Conclusion
The overall antibody response to PCV13 is adequate in patients with gastric and colorectal cancer during adjuvant chemotherapy, and no significant difference was found when patients were vaccinated two weeks before or on the day of chemotherapy initiation.
Figure
Cited by 1 articles
-
Impact of a Doctor's Verbal Recommendation on Pneumococcal 13-Valent Conjugate Vaccine among High-Risk Patients for Pneumonia in a Primary Care Setting
Jihan Kim, Sami Lee, Jong Sung Kim
Korean J Health Promot. 2021;21(1):8-16. doi: 10.15384/kjhp.2021.21.1.8.
Reference
-
References
1. Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014; 58:e44–100.
Article2. Shildt RA, Boyd JF, McCracken JD, Schiffman G, Giolma JP. Antibody response to pneumococcal vaccine in patients with solid tumors and lymphomas. Med Pediatr Oncol. 1983; 11:305–9.
Article3. Nordoy T, Aaberge IS, Husebekk A, Samdal HH, Steinert S, Melby H, et al. Cancer patients undergoing chemotherapy show adequate serological response to vaccinations against influenza virus and Streptococcus pneumoniae. Med Oncol. 2002; 19:71–8.4. Hung TY, Kotecha RS, Blyth CC, Steed SK, Thornton RB, Ryan AL, et al. Immunogenicity and safety of single-dose, 13-valent pneumococcal conjugate vaccine in pediatric and adolescent oncology patients. Cancer. 2017; 123:4215–23.
Article5. Wumkes ML, van der Velden AM, Los M, Leys MB, Beeker A, Nijziel MR, et al. Serum antibody response to influenza virus vaccination during chemotherapy treatment in adult patients with solid tumours. Vaccine. 2013; 31:6177–84.
Article6. Rodgers GL, Esposito S, Principi N, Gutierrez-Brito M, Diez-Domingo J, Pollard AJ, et al. Immune response to 13-valent pneumococcal conjugate vaccine with a reduced dosing schedule. Vaccine. 2013; 31:4765–74.
Article7. Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016; 4:399–406.
Article8. Song JY, Nahm MH, Moseley MA. Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci. 2013; 28:4–15.
Article9. Song JY, Cheong HJ, Tsai TF, Chang HA, Choi MJ, Jeon JH, et al. Immunogenicity and safety of concomitant MF59-adjuvanted influenza vaccine and 23-valent pneumococcal polysaccharide vaccine administration in older adults. Vaccine. 2015; 33:4647–52.
Article10. Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006; 13:1004–9.
Article11. Beyer WE, Palache AM, Luchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004; 103:125–32.
Article12. Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013; 19:412–25.
Article13. Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999; 29:281–8.
Article14. Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis. 2003; 187:1639–45.
Article15. Durando P, Faust SN, Fletcher M, Krizova P, Torres A, Welte T. Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults. Clin Microbiol Infect. 2013; 19 Suppl 1:1–9.
Article16. Addiego JE Jr, Ammann AJ, Schiffman G, Baehner R, Higgins G, Hammond D. Response to pneumococcal polysaccharide vaccine in patients with untreated Hodgkin's disease: Children's Cancer Study Group Report. Lancet. 1980; 2:450–2.17. Frederiksen B, Specht L, Henrichsen J, Pedersen FK, Pedersen-Bjergaard J. Antibody response to pneumococcal vaccine in patients with early stage Hodgkin's disease. Eur J Haematol. 1989; 43:45–9.
Article18. Sinisalo M, Vilpo J, Itala M, Vakevainen M, Taurio J, Aittoniemi J. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine. 2007; 26:82–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of 7-valent pneumococcal conjugate vaccine related to booster immunization in Korean children
- Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines
- Indirect Effects of Pneumococcal Conjugate Vaccines in National Immunization Programs for Children on Adult Pneumococcal Disease
- Immunogenicity and safety of a 12-valent pneumococcal conjugate vaccine in infants aged 6–10 weeks: a randomized double-blind active-controlled trial
- Pneumococcal vaccine