Cancer Res Treat.
2020 Apr;52(2):604-621. 10.4143/crt.2019.444.
Activation of Tyrosine Metabolism in CD13+ Cancer Stem Cells DrivesRelapse in Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Oncology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
- 2The Nursing Department, Shanghai Public Health Clinical Center, Shanghai, China
- 3The First Department of Infectious Diseases, Shanghai Public Health Clinical Center, Shanghai, China
- 4The Third Department of Infectious Diseases, Shanghai Public Health Clinical Center, Shanghai, China
- KMID: 2500345
- DOI: http://doi.org/10.4143/crt.2019.444
Abstract
- Purpose
Cancer stem cells (CSCs) are naturally resistant to chemotherapy, explaining why tumor relapse frequently occurs after initial regression upon administration of chemotherapeutic agents in most cases. A CSC population characterized by CD13 expression has been identified in hepatocellular carcinoma (HCC). In the current study, we aimed to clarify the molecular mechanism by which it escapes conventional therapies.
Materials and Methods
Here, we used flow cytometry to examine the percentage of CD13+ CSCs in HepG2 and HuH7 cells after chemotherapy. Using in vitro isotope labeling technique, we compared metabolic pathways between CD13+ and CD13– subpopulations. Using co-immunoprecipitation and western blotting, we determined the target expressions in protein levels under different conditions. We also performed immunohistochemistry to detect the target proteins under different conditions. Animal models were constructed to verify the potential role of tyrosine metabolism in post-chemotherapeutic relapse in vivo.
Results
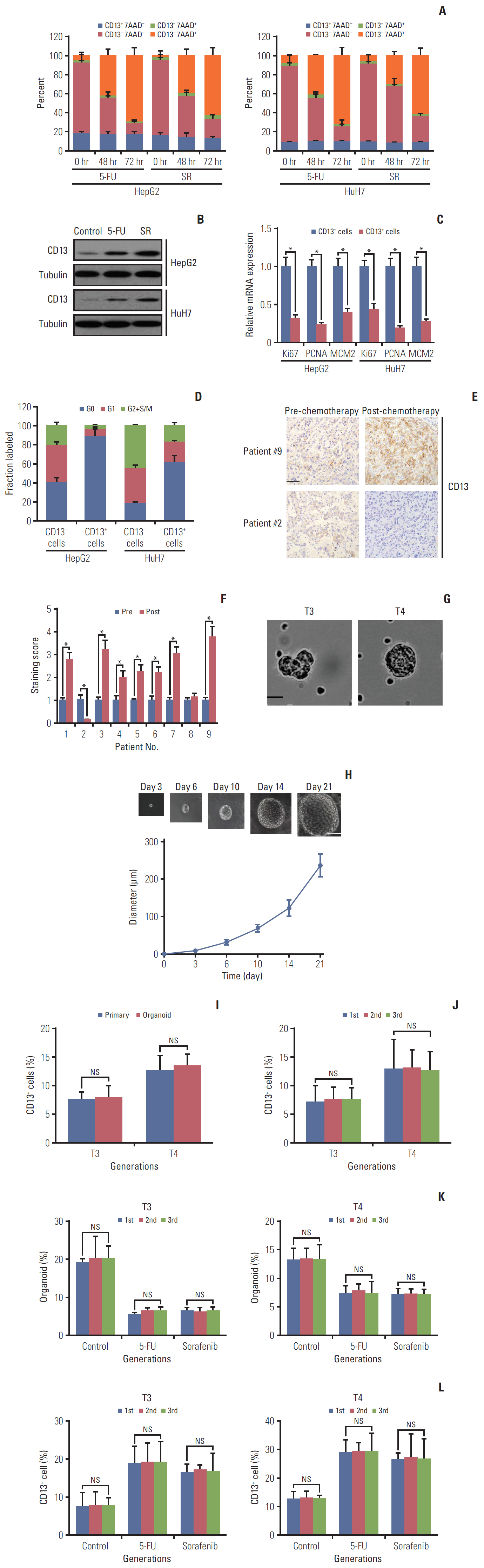
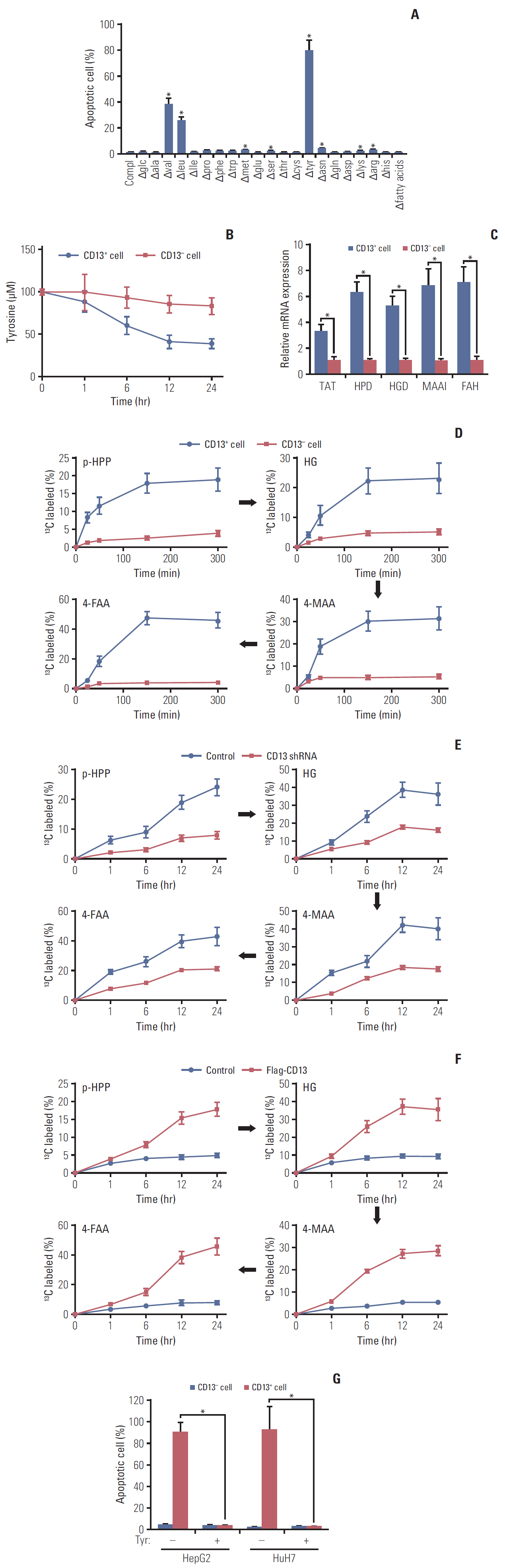
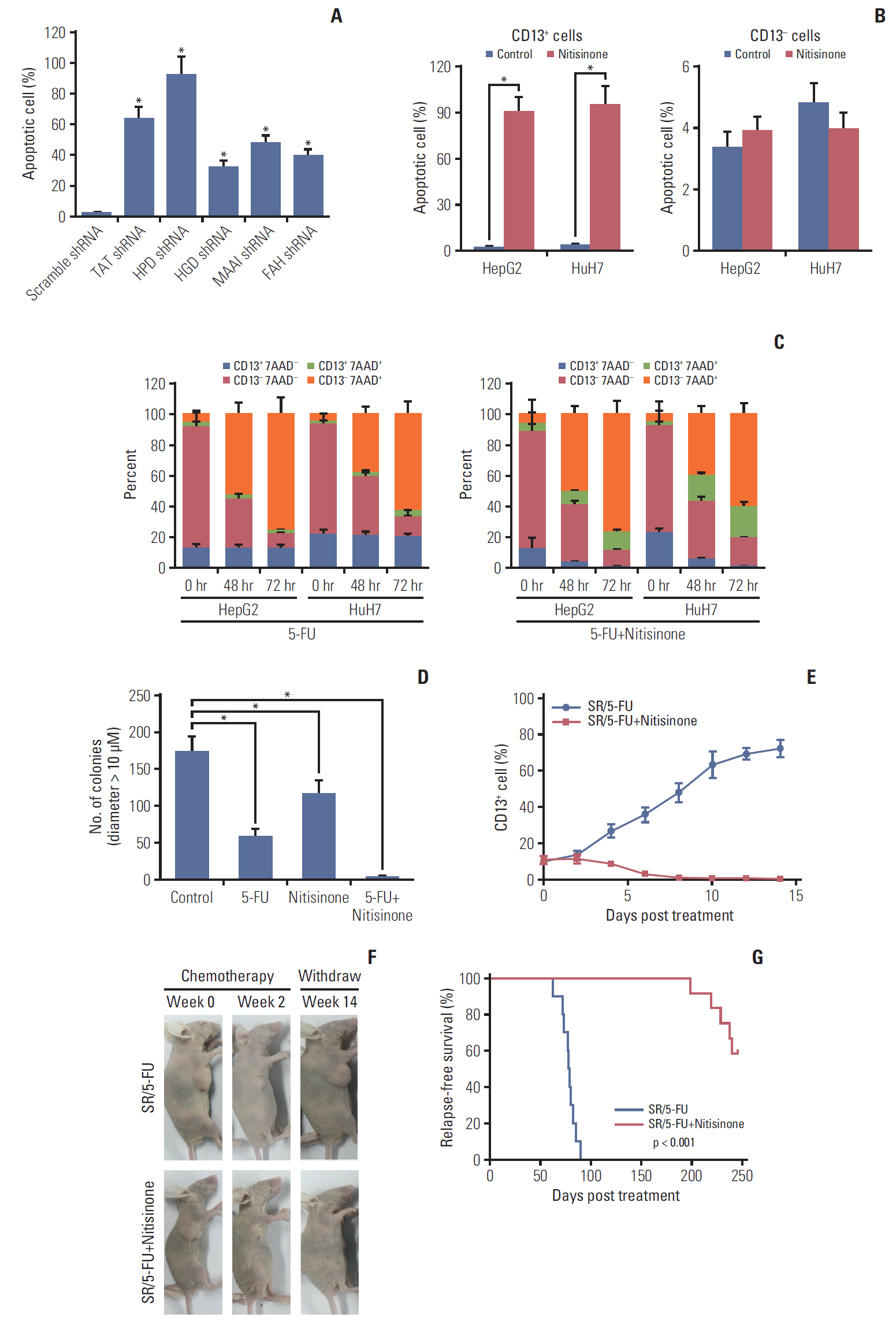
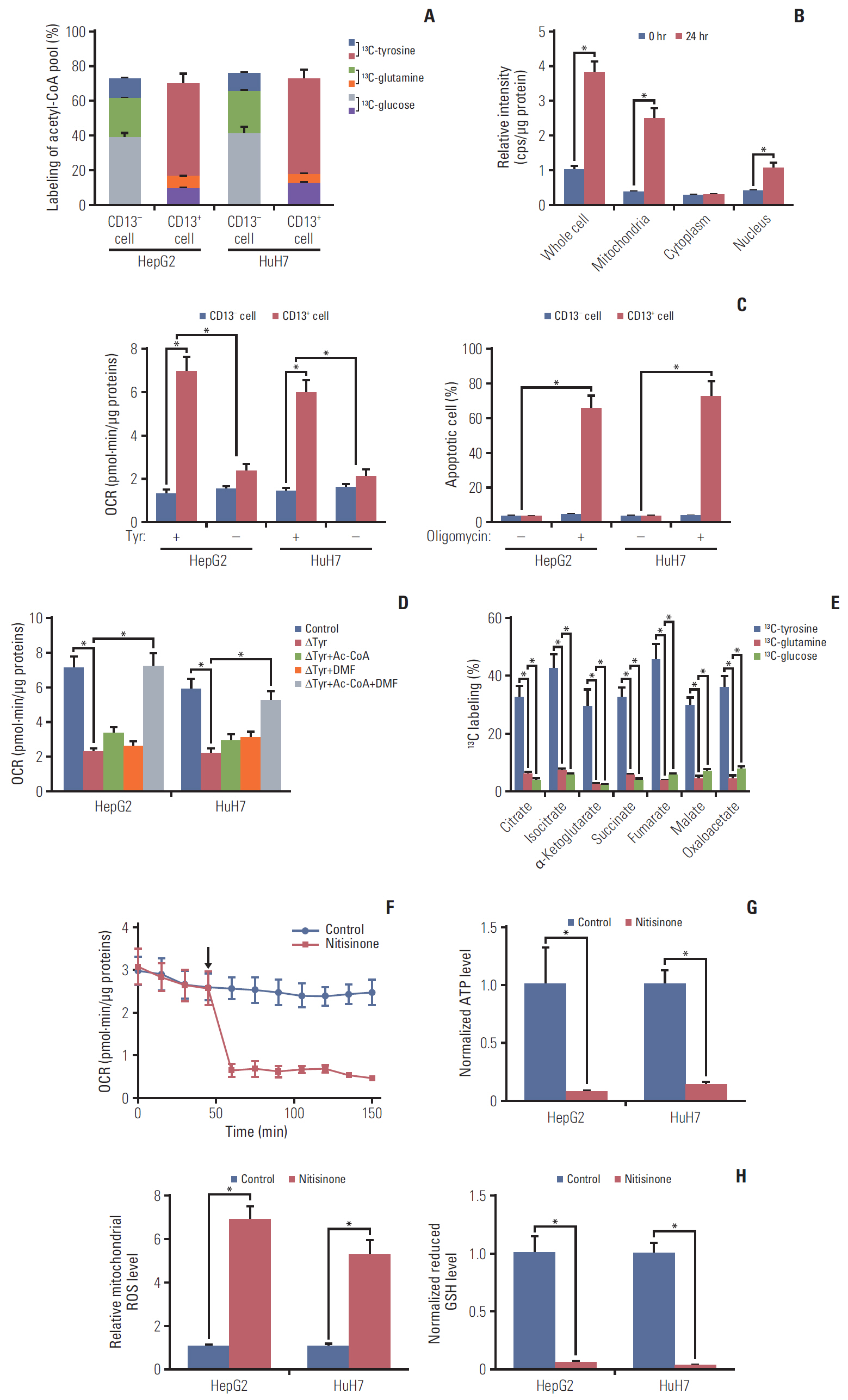
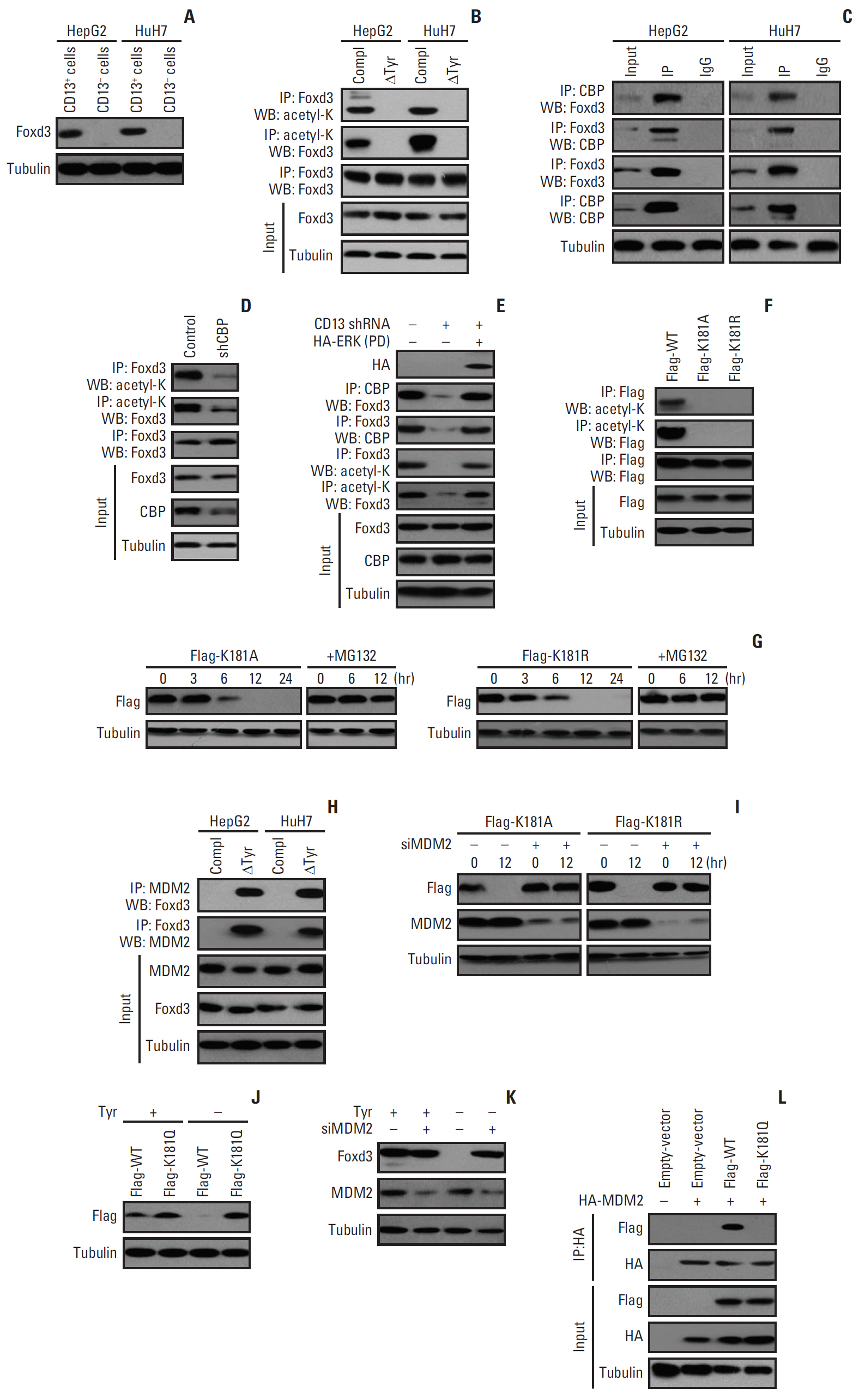
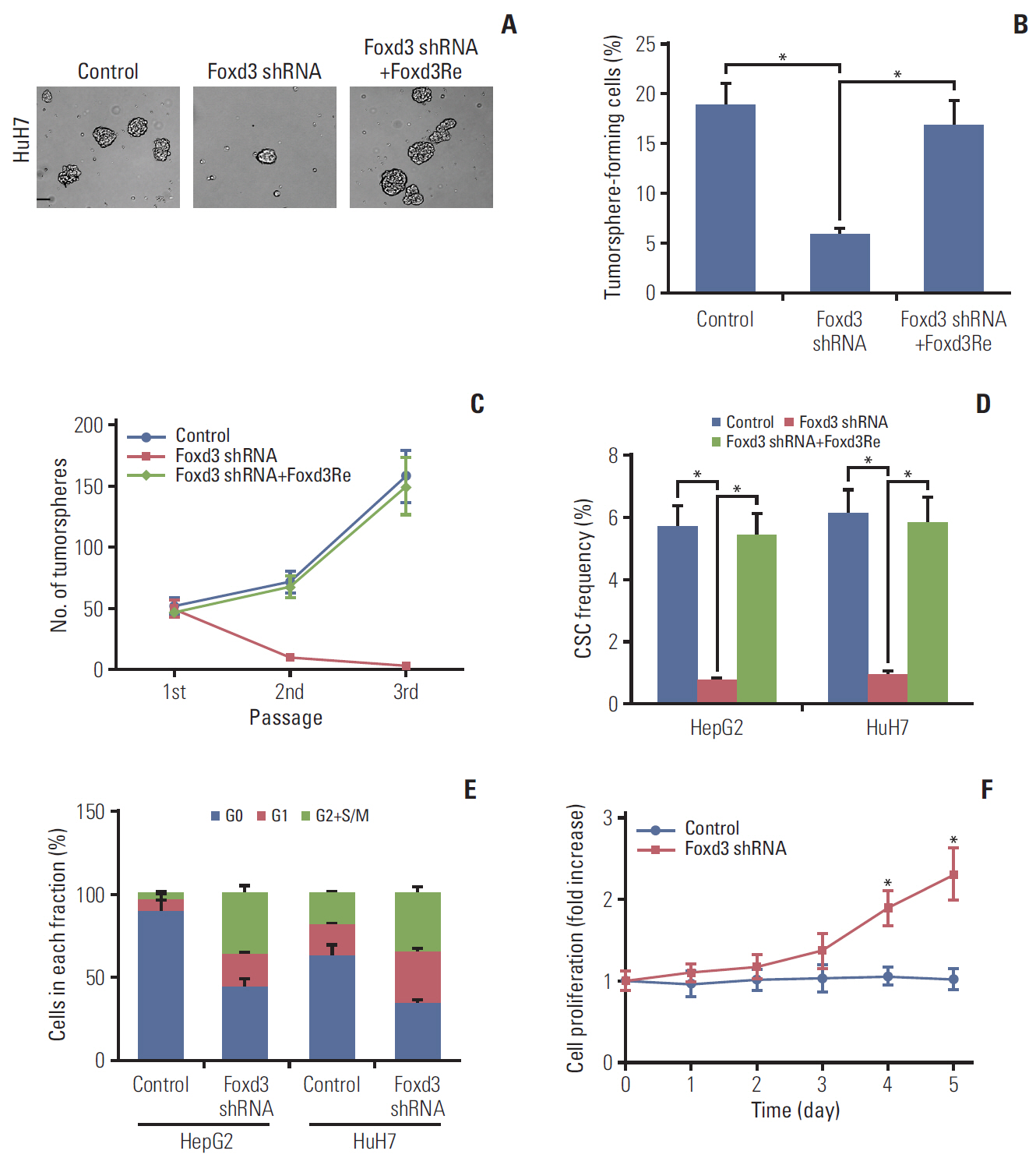
We observed that quiescent CD13+ CSCs are enriched after chemotherapy in HCCs, and serve as a reservoir for recurrence. Mechanistically, CD13+ CSCs were dependent on aerobic metabolism of tyrosine rather than glucose as energy source. Tyrosine metabolism also generated nuclear acetyl-CoA to acetylate and stabilize Foxd3, thereby allowing CD13+ CSCs cells to sustain quiescence and resistance to chemotherapeutic agents.
Conclusion
These findings encourage further exploration of eliminating CD13+ cells by targeting specific metabolic pathways to prevent recurrence in HCCs.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–2.
Article3. European Association for the Study of the Liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018; 69:154–81.4. Phi LT, Sari IN, Yang YG, Lee SH, Jun N, Kim KS, et al. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018; 2018:541–6923.
Article5. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013; 123:1911–8.
Article6. Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008; 47:919–28.
Article7. Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007; 132:2542–56.
Article8. Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Invest. 2010; 120:3326–39.
Article9. Christ B, Stock P, Dollinger MM. CD13: waving the flag for a novel cancer stem cell target. Hepatology. 2011; 53:1388–90.
Article10. Luan Y, Xu W. The structure and main functions of aminopeptidase N. Curr Med Chem. 2007; 14:639–47.
Article11. Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. Br J Cancer. 2016; 114:1305–12.
Article12. Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. Cancer stem cell metabolism: a potential target for cancer therapy. Mol Cancer. 2016; 15:69.
Article13. Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011; 11:85–95.
Article14. Li L, Bhatia R. Stem cell quiescence. Clin Cancer Res. 2011; 17:4936–41.15. Drost J, Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018; 18:407–18.
Article16. Mao P, Joshi K, Li J, Kim SH, Li P, Santana-Santos L, et al. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A. 2013; 110:8644–9.
Article17. Ferreira LM, Hebrant A, Dumont JE. Metabolic reprogramming of the tumor. Oncogene. 2012; 31:3999–4011.
Article18. Lock E, Ranganath LR, Timmis O. The role of nitisinone in tyrosine pathway disorders. Curr Rheumatol Rep. 2014; 16:457.
Article19. Ait-Si-Ali S, Carlisi D, Ramirez S, Upegui-Gonzalez LC, Duquet A, Robin P, et al. Phosphorylation by p44 MAP Kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem Biophys Res Commun. 1999; 262:157–62.
Article20. Santos AN, Langner J, Herrmann M, Riemann D. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Cell Immunol. 2000; 201:22–32.
Article21. Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008; 10:138–48.
Article22. Pauklin S, Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013; 155:135–47.
Article23. Merlos-Suarez A, Barriga FM, Jung P, Iglesias M, Cespedes MV, Rossell D, et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell. 2011; 8:511–24.
Article24. Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014; 15:243–56.
Article25. Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011; 476:346–50.
Article26. Wensveen FM, Alves NL, Derks IA, Reedquist KA, Eldering E. Apoptosis induced by overall metabolic stress converges on the Bcl-2 family proteins Noxa and Mcl-1. Apoptosis. 2011; 16:708–21.
Article27. Kim HM, Haraguchi N, Ishii H, Ohkuma M, Okano M, Mimori K, et al. Increased CD13 expression reduces reactive oxygen species, promoting survival of liver cancer stem cells via an epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol. 2012; 19 Suppl 3:S539–48.
Article28. Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nat Rev Mol Cell Biol. 2012; 13:270–6.
Article29. Yong JS, Intriago-Baldeon DP, Lam EW. FOXD3 controls pluripotency through modulating enhancer activity. Stem Cell Investig. 2016; 3:17.
Article30. Stewart RM, Briggs MC, Jarvis JC, Gallagher JA, Ranganath L. Reversible keratopathy due to hypertyrosinaemia following intermittent low-dose nitisinone in alkaptonuria: a case report. JIMD Rep. 2014; 17:1–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cellular origin of liver cancer stem cells
- Activation of PKCdelta by tyrosine phosphorylation in rat parotid acinar cells
- Knockdown of 14-3-3zeta enhances radiosensitivity and radio-induced apoptosis in CD133+ liver cancer stem cells
- Expression of CD133, CD44, CK7, and OCT4 in Animal Cancers
- D60-sensitive tyrosine phosphorylation is involved in Fas-mediated phospholipase D activation