Blood Res.
2020 Mar;55(1):27-34. 10.5045/br.2020.55.1.27.
Similar transplant outcomes between haploidentical and unrelated donors after reduced-intensity conditioning with busulfan, fludarabine, and anti-thymocyte globulin in patients with acute leukemia or myelodysplastic syndrome
- Affiliations
-
- 1Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. stephano.dyshin@gmail.com
- 2Center for Medical Innovation, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2472005
- DOI: http://doi.org/10.5045/br.2020.55.1.27
Abstract
- BACKGROUND
Although T-cell-replete hematopoietic cell transplantation (HCT) from haploidentical donors (HIDs) using anti-thymocyte globulin (ATG) has shown promising outcomes, previous studies often adopted heterogenous graft sources and conditioning.
METHODS
We retrospectively compared HCT outcomes from 62 HIDs, 36 partially-matched unrelated donors (PUDs), and 55 matched unrelated donors (MUDs) in patients with acute leukemia or myelodysplastic syndrome using the same graft source of peripheral blood and a reduced intensity conditioning of busulfan, fludarabine, and ATG.
RESULTS
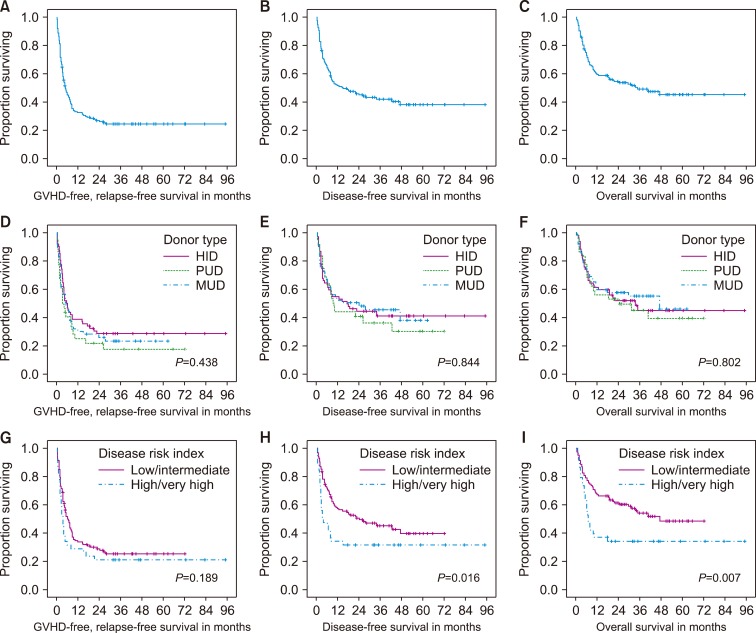
The estimates of 3-yr disease-free survival (DFS) and overall survival (OS) rates were not significantly different among the MUD, HID, and PUD groups, at 46%, "41%, and 36%" for the DFS rate (P=0.844), and 55%, 45%, and 45% for the OS rate (P=0.802), respectively. Cumulative incidence of relapse and non-relapse mortality at 3 yr was similar among different donor types. Subsequent multivariable analyses showed that the sex of the patient (male) and a high/very high disease risk index were independently associated with poorer DFS and OS, while the donor type was not.
CONCLUSION
T-cell replete HCT from HIDs using an ATG-containing reduced intensity conditioning regimen may be a reasonable option in the absence of matched related donors in patients with acute leukemia or myelodysplastic syndrome.
Keyword
MeSH Terms
Figure
Reference
-
1. Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998; 339:1177–1185. PMID: 9780337.2. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006; 354:1813–1826. PMID: 16641398.
Article3. Barker JN, Krepski TP, DeFor TE, Davies SM, Wagner JE, Weisdorf DJ. Searching for unrelated donor hematopoietic stem cells: availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002; 8:257–260. PMID: 12064362.
Article4. Ciurea SO, Bayraktar UD. "No donor"? Consider a haploidentical transplant. Blood Rev. 2015; 29:63–70. PMID: 25307958.
Article5. Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983; 1:612–615. PMID: 6131300.
Article6. Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985; 313:765–771. PMID: 3897863.
Article7. Passweg JR, Baldomero H, Bader P, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017; 52:811–817. PMID: 28287639.
Article8. Luo Y, Xiao H, Lai X, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014; 124:2735–2743. PMID: 25214441.
Article9. Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18:295–304. PMID: 4153799.
Article10. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11:945–956. PMID: 16338616.11. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106:2912–2919. PMID: 15994282.
Article12. Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014; 123:3664–3671. PMID: 24744269.
Article13. Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015; 125:1333–1338. PMID: 25593335.
Article14. Solh M, Zhang X, Connor K, et al. Factors predicting graftversus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation: multivariable analysis from a single center. Biol Blood Marrow Transplant. 2016; 22:1403–1409. PMID: 27095692.
Article15. Piemontese S, Ciceri F, Labopin M, et al. A comparison between allogeneic stem cell transplantation from unmanipulated haploidentical and unrelated donors in acute leukemia. J Hematol Oncol. 2017; 10:24. PMID: 28103944.
Article16. McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015; 125:3024–3031. PMID: 25814532.
Article17. Kim HT, Zhang MJ, Woolfrey AE, et al. Donor and recipient sex in allogeneic stem cell transplantation: what really matters. Haematologica. 2016; 101:1260–1266. PMID: 27354023.
Article18. Socié G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011; 117:6375–6382. PMID: 21467544.
Article19. Kröger N, Solano C, Bonifazi F. Antilymphocyte globulin for chronic graft-versus-host disease. N Engl J Med. 2016; 374:1894–1895.
Article20. Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013; 121:849–857. PMID: 23165479.
Article21. Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood. 2015; 125:3956–3962. PMID: 25940714.
Article22. Lee KH, Lee JH, Lee JH, et al. Reduced-intensity conditioning therapy with busulfan, fludarabine, and antithymocyte globulin for HLA-haploidentical hematopoietic cell transplantation in acute leukemia and myelodysplastic syndrome. Blood. 2011; 118:2609–2617. PMID: 21715313.
Article23. Lee KH, Lee JH, Lee JH, et al. Reduced-intensity conditioning with busulfan, fludarabine, and antithymocyte globulin for hematopoietic cell transplantation from unrelated or haploidentical family donors in patients with acute myeloid leukemia in remission. Biol Blood Marrow Transplant. 2017; 23:1555–1566. PMID: 28552421.
Article24. Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014; 20:1573–1579. PMID: 24910379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Treosulfan-Based Conditioning Attractive as a Reduced-Intensity Conditioning Regimen in Korea?
- Differential impact of anti-thymocyte globulin dosing by disease risk index in alternative donor peripheral blood stem cell transplantation in patients with acute leukemia or myelodysplastic syndrome after reduced intensity conditioning
- Unrelated Bone Marrow Transplantation with a Reduced Toxicity Myeloablative Conditioning Regimen in Wiskott-Aldrich Syndrome
- Does anti-thymocyte globulin have a place in busulfan/fludarabine conditioning for matched related donor hematopoietic stem cell transplantation?
- Haplotype Mismatch Transplantation using High-dose CD34+ Cells with Stratified New Conditioning Regimens in Patients with Acute Myeloid Leukemia