World J Mens Health.
2020 Apr;38(2):226-235. 10.5534/wjmh.190029.
Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy
- Affiliations
-
- 1Department of Urology, Kyungpook National University Chilgok Hospital, Daegu, Korea.
- 2Department of Urology, Kyungpook National University Hospital, Daegu, Korea. skhwan@gmail.com
- 3Department of Urology, School of Medicine, Kyungpook National University, Daegu, Korea.
- 4Department of Urology, Jikei University School of Medicine, Tokyo, Japan.
- KMID: 2471829
- DOI: http://doi.org/10.5534/wjmh.190029
Abstract
- PURPOSE
The purpose of this study was to determine the comparative effectiveness of androgen deprivation therapy (ADT) combined with docetaxel (DTX)-based chemotherapy in Korean and Japanese castration-resistant prostate cancer (CRPC) patient cohorts.
MATERIALS AND METHODS
Metastatic CRPC patients who underwent more than three DTX-based chemotherapy cycles in Korea and Japan between 2002 and 2017 were retrospectively analyzed and divided into the DTX-only (DTX, n=30) and combination (DTX+ADT, n=46) groups. Progression-free survival (PFS) was calculated as the time from the start of chemotherapy to the occurrence of either disease progression (prostate-specific antigen [PSA] progression or radiographic progression) or death. The primary end point was PFS and the secondary end point was overall survival (OS).
RESULTS
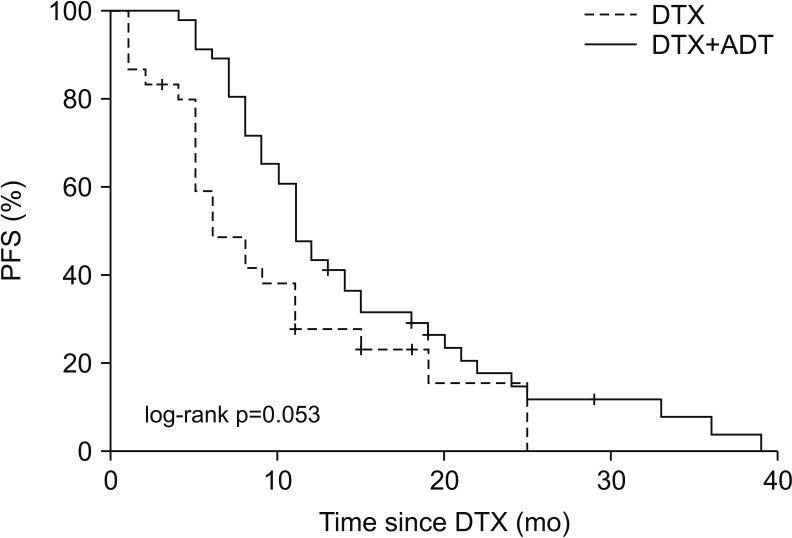
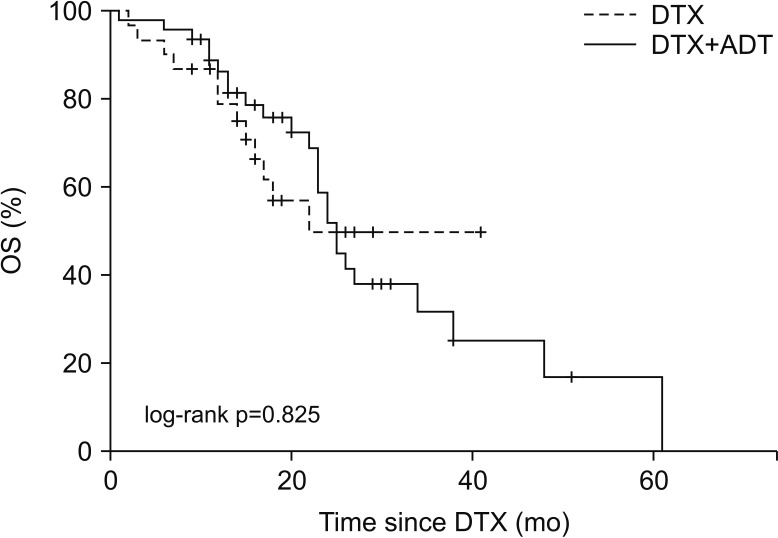
In the DTX and DTX+ADT groups, the median PFS was 6.0 and 11.0 months (log-rank p=0.053). The multivariate Cox regression analysis revealed that the significant predicting factors of PFS were ADT administration (hazard ratio [HR], 0.478; 95% confidence interval [CI], 0.284-0.804; p=0.005) and number of DTX-based chemotherapy cycles (HR, 0.934; 95% CI, 0.899-0.970; p<0.001). In the DTX and DTX+ADT groups, the median OS was 16.0 and 19.5 months (log-rank p=0.825). Through multiple Cox regression analysis, we found that the significant predicting factors of OS were the PSA nadir level (HR, 1.001; 95% CI, 1.000-1.002; p<0.001) and number of DTX-based chemotherapy cycles (HR, 0.932; 95% CI, 0.876-0.991; p=0.024).
CONCLUSIONS
Concurrent DTX-based chemotherapy and ADT may be beneficial compared with DTX-based chemotherapy alone in chemotherapy-naïve metastatic CRPC patients in terms of the PFS, but not the OS.
MeSH Terms
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30. PMID: 26742998.
Article2. Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int J Urol. 2018; 25:524–531. PMID: 29740894.
Article3. Jung KW, Won YJ, Kong HJ, Lee ES. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018; 50:303–316. PMID: 29566481.
Article4. Kakehi Y, Sugimoto M, Taoka R. Committee for Establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition). Int J Urol. 2017; 24:648–666. PMID: 28667698.
Article5. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008; 26:1148–1159. PMID: 18309951.
Article6. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004; 351:1513–1520. PMID: 15470214.
Article7. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351:1502–1512. PMID: 15470213.
Article8. Hussain M, Wolf M, Marshall E, Crawford ED, Eisenberger M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol. 1994; 12:1868–1875. PMID: 8083710.
Article9. Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993; 11:2167–2172. PMID: 8229130.
Article10. Scher HI, Buchanan G, Gerald W, Butler LM, Tilley WD. Targeting the androgen receptor: improving outcomes for castration-resistant prostate cancer. Endocr Relat Cancer. 2004; 11:459–476. PMID: 15369448.
Article11. Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989; 321:419–424. PMID: 2503724.
Article12. Wong YN, Freedland S, Egleston B, Hudes G, Schwartz JS, Armstrong K. Role of androgen deprivation therapy for node-positive prostate cancer. J Clin Oncol. 2009; 27:100–105. PMID: 19047295.
Article13. Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008; 300:173–181. PMID: 18612114.
Article14. Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005; 294:238–244. PMID: 16014598.
Article15. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999; 341:1781–1788. PMID: 10588962.
Article16. Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Eastern Cooperative Oncology Group study EST 3886. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006; 7:472–479. PMID: 16750497.
Article17. Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002; 360:103–106. PMID: 12126818.
Article18. Holzbeierlein JM, McLaughlin MD, Thrasher JB. Complications of androgen deprivation therapy for prostate cancer. Curr Opin Urol. 2004; 14:177–183. PMID: 15069309.
Article19. Weight CJ, Klein EA, Jones JS. Androgen deprivation falls as orchiectomy rates rise after changes in reimbursement in the U.S. Medicare population. Cancer. 2008; 112:2195–2201. PMID: 18393326.
Article20. Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013; 32:5501–5511. PMID: 23752182.
Article21. Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009; 6:76–85. PMID: 19198621.
Article22. Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001; 1:34–45. PMID: 11900250.
Article23. Virgo KS, Basch E, Loblaw DA, Oliver TK, Rumble RB, Carducci MA, et al. Second-line hormonal therapy for men with chemotherapy-naïve, castration-resistant prostate cancer: American Society of Clinical Oncology provisional clinical opinion. J Clin Oncol. 2017; 35:1952–1964. PMID: 28441112.
Article24. Jang HS, Koo KC, Cho KS, Chung BH. Survival outcomes of concurrent treatment with docetaxel and androgen deprivation therapy in metastatic castration-resistant prostate cancer. Yonsei Med J. 2016; 57:1070–1078. PMID: 27401636.
Article25. Lee DH, Kim JH, Seo WI, Nam JK, Kim TN, Oh CK, et al. Clinical outcomes of continuous addition of androgen deprivation therapy during docetaxel chemotherapy for patients with castration-resistant prostate cancer. Korean J Urol Oncol. 2017; 15:59–65.
Article26. Lee JL, Eun Kim J, Ahn JH, Lee DH, Lee J, Kim CS, et al. Role of androgen deprivation treatment in patients with castration-resistant prostate cancer, receiving docetaxel-based chemotherapy. Am J Clin Oncol. 2011; 34:140–144. PMID: 20686407.
Article27. Nam W, Choi SY, Yoo SJ, Ryu J, Lee J, Kyung YS, et al. Factors associated with testosterone recovery after androgen deprivation therapy in patients with prostate cancer. Investig Clin Urol. 2018; 59:18–24.
Article28. Nejat RJ, Rashid HH, Bagiella E, Katz AE, Benson MC. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. J Urol. 2000; 164:1891–1894. PMID: 11061874.
Article29. D'Amico AV, Renshaw AA, Loffredo B, Chen MH. Duration of testosterone suppression and the risk of death from prostate cancer in men treated using radiation and 6 months of hormone therapy. Cancer. 2007; 110:1723–1728. PMID: 17828774.30. de Morrée ES, Vogelzang NJ, Petrylak DP, Budnik N, Wiechno PJ, Sternberg CN, et al. Association of survival benefit with docetaxel in prostate cancer and total number of cycles administered: a post hoc analysis of the mainsail study. JAMA Oncol. 2017; 3:68–75. PMID: 27560549.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Systemic Therapies for Metastatic Castration-Resistant Prostate Cancer: An Updated Review
- Hormonal therapy and chemotherapy for advanced prostate cancer
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- Current Treatment Strategies for Castration-Resistant Prostate Cancer