Korean J Gastroenterol.
2020 Jan;75(1):39-45. 10.4166/kjg.2020.75.1.39.
Effect of Sodium Cromoglycate on Acetic Acid-induced Ulcerative Colitis in Mice
- Affiliations
-
- 1Department of Biochemistry, Biophysics and Genetics, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
- 2Department of Internal Medicine, School of Medicine, Alimentary Tract Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 3Department of Pharmacology, School of Medicine, Yasuj University of Medical Science, Yasuj, Iran.
- 4Clinical Microbiology Research Center, Ilam University of Medical Sciences, Ilam, Iran.
- 5Laboratory Sciences Research Center, Golestan University of Medical Sciences, Gorgan, Iran.
- 6Department of Pharmacology, School of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
- 7Department of Pharmacology, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. dr.houshmand_pharmaco@yahoo.com
- 8Gut and Liver Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
- KMID: 2471138
- DOI: http://doi.org/10.4166/kjg.2020.75.1.39
Abstract
- BACKGROUND/AIMS
Ulcerative colitis (UC) is a type of inflammatory bowel disease that mainly involves the colon. Thus far, glucocorticoids and amino-salicylate have been the main treatment.
METHODS
To assess drugs with fewer side effects, this study evaluated the effects of sodium cromoglycate (SCG) on acetic acid-induced UC in rats. The treatment groups included SCG receivers (50 and 100 mg/kg, intra-orally) and sulfasalazine (SSZ) receivers (100 mg/kg, intra-orally). The colonic mucosal injury was assessed by clinical, macroscopic, and histopathological examinations.
RESULTS
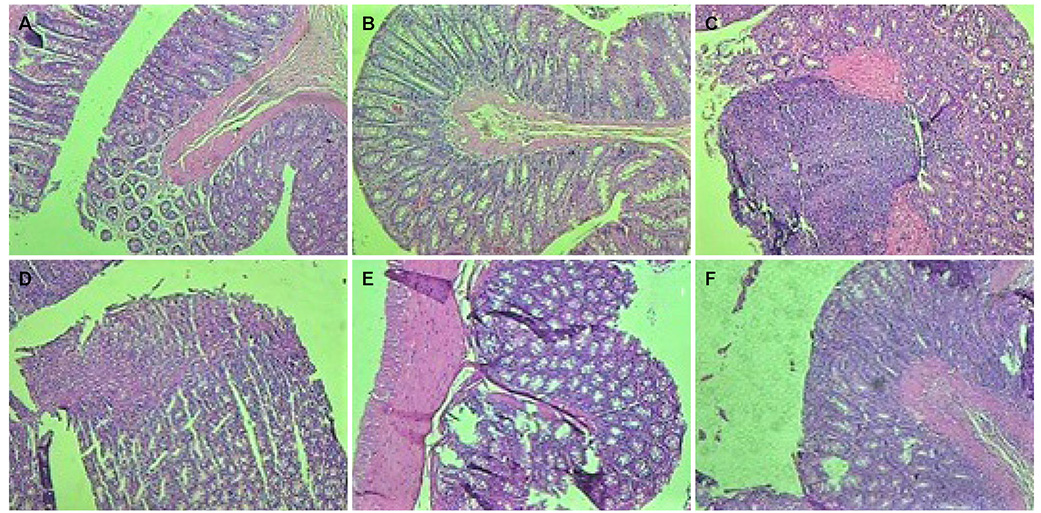
In the treatment groups with 50 and 100 mg/kg of SCG, the clinical activity score decreased to 2.67±0.18 and 1.73±0.21 (p<0.05), respectively, compared to the UC control group (3.21±0.31), and were higher than that of the group given the standard treatment of 100 mg/kg SSZ (1.10±0.09). The treatment groups with 50 and 100 mg/kg of SCG showed a lower clinical gross lesion score than the UC control group (2.91±0.28 and 2.10±0.43, vs. 4.49±0.61, p<0.05) and were higher than the standard group (0.95±0.18). Treatment with SCG (100 mg/kg) decreased the macroscopic scores significantly compared to the UC control group (p<0.05) on the 8th day.
CONCLUSIONS
SCG (100mg/kg) decreased significantly the clinical activity score, gross lesion, and percentage-affected area compared to the UC controls on the 8th day.
MeSH Terms
Figure
Reference
-
1. Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014; 7:113–120.
Article2. Li J, Ueno A, Fort Gasia M, et al. Profiles of lamina propria T helper cell subsets discriminate between ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2016; 22:1779–1792.
Article3. Shivashankar R, Lichtenstein GR. Mimics of inflammatory bowel disease. Inflamm Bowel Dis. 2018; 24:2315–2321.
Article4. Asakura H, Kitahora T. Antioxidants and polyphenols in inflammatory bowel disease: ulcerative colitis and Crohn disease. In : Watson RR, Preedy VR, Zibadi S, editors. Polyphenols: prevention and treatment of human disease. Volume 2. 2nd ed. Cambridge (MA): Academic Press;2018. p. 279–292.5. Stasikowska-Kanicka O, Danilewicz M, Głowacka A, Wągrowska-Danilewicz M. Mast cells and eosinophils are involved in activation of ulcerative colitis. Adv Med Sci. 2012; 57:230–236.
Article6. Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017; 12:1295–1309.
Article7. Bonovas S, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2018; 47:454–465.
Article8. Taylor K, Gibson PR. Conventional therapy of ulcerative colitis: corticosteroids. In : Baumgart DC, editor. Crohn's disease and ulcerative colitis. 2nd ed. Melbourne: Springer;2017. p. 399–412.9. Sravani MR, Naveen A. Anti-ulcer effect of sodium cromoglycate in NSAID and ethanol induced ulcer in comparison with ranitidine in rats. Pharma Innov. 2017; 6:1–5.10. Roviezzo F, Sorrentino R, Iacono VM, et al. Disodium cromoglycate inhibits asthma-like features induced by sphingosine-1-phosphate. Pharmacol Res. 2016; 113(Pt A):626–635.
Article11. Bischoff SC. Mast cells in gastrointestinal disorders. Eur J Pharmacol. 2016; 778:139–145.
Article12. Lieberman JA, Zhang J, Whitworth J, Cavender C. A randomized, double-blinded, placebo-controlled study of the use of viscous oral cromolyn sodium for the treatment of eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2018; 120:527–531.
Article13. Cohen RD. Inflammatory bowel disease: diagnosis and therapeutics. 1st ed. Totowa (NJ): Humana Press;2003.14. Henriksen M, Høivik ML, Jelsness-Jørgensen LP, Moum B. IBSEN Study Group. Irritable bowel-like symptoms in ulcerative colitis are as common in patients in deep remission as in inflammation: results from a population-based study [the IBSEN study]. J Crohns Colitis. 2018; 12:389–393.
Article15. Khan I, Samson SE, Grover AK. Antioxidant supplements and gastrointestinal diseases: a critical appraisal. Med Princ Pract. 2017; 26:201–217.
Article16. Vasudevan A, Gibson PR, van Langenberg DR. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: what should the clinician expect, what should patients be told? World J Gastroenterol. 2017; 23:6385–6402.
Article17. Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008; 135:1907–1913.
Article18. Kotze PG, Albuquerque IC, Moraes AC, Vieira A, Souza F. Análise de custo-minimização entre o infliximabe (IFX) e o adalimumabe (ADA) no tratamento da doença de Crohn (DC). Rev Bras Coloproct. 2009; 29:158–168.
Article19. Shailubhai K, Palejwala V, Arjunan KP, et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 2015; 6:213–222.
Article20. Solberg IC, Lygren I, Jahnsen J, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN study). Scand J Gastroenterol. 2009; 44:431–440.
Article21. Shi Y, Liu H, Huang Z, Liu Z. Clinical effects of sulfasalazine combined with live combined bifidobacterium lactobacillus and enterococcus capsules in patients with ulcerative colitis. Chinese Journal of New Drugs and Clinical Remedies. 2010; 10:783–785.22. Shin MR, Kim KJ, Kim SH, et al. Comparative evaluation between sulfasalazine alone and in combination with herbal medicine on DSS-induced ulcerative colitis mice. Biomed Res Int. 2017; 2017:6742652.
Article23. Rintala RJ, Lindahl H. Sodium cromoglycate in the management of chronic or recurrent enterocolitis in patients with Hirschsprung's disease. J Pediatr Surg. 2001; 36:1032–1035.
Article24. Srivastava V, Viswanathaswamy AH, Mohan G. Determination of the antiulcer properties of sodium cromoglycate in pylorus-ligated albino rats. Indian J Pharmacol. 2010; 42:185–188.
Article25. Doherty GA, Cheifetz AS. Management of acute severe ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2009; 3:395–405.
Article26. Jiminez JA, Uwiera TC, Douglas Inglis G, Uwiera RR. Animal models to study acute and chronic intestinal inflammation in mammals. Gut Pathog. 2015; 7:29.
Article27. Kopecki Z, Yang G, Treloar S, et al. Flightless I exacerbation of inflammatory responses contributes to increased colonic damage in a mouse model of dextran sulphate sodium-induced ulcerative colitis. Sci Rep. 2019; 9:12792.
Article28. Yokooji T, Matsuo H. Sodium cromoglycate prevents exacerbation of IgE-mediated food-allergic reaction induced by aspirin in a rat model of egg allergy. Int Arch Allergy Immunol. 2015; 167:193–202.
Article29. Stoyanova II, Gulubova MV. Mast cells and inflammatory mediators in chronic ulcerative colitis. Acta Histochem. 2002; 104:185–192.
Article30. Jiang L, Fang P, Septer S, Apte U, Pritchard MT. Inhibition of mast cell degranulation with cromolyn sodium exhibits organ-specific effects in polycystic kidney (PCK) rats. Int J Toxicol. 2018; 37:308–326.
Article31. Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn’s effectiveness and selectivity as a ‘mast cell stabilizer' in mice. Lab Invest. 2012; 92:1472–1482.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents
- Animal Models of Inflammatory Bowel Disease
- Correction: Effect of Sodium Cromoglycate on Acetic Acid-induced Ulcerative Colitis in Mice
- 5-Aminosalicylic Acid-induced Myocarditis in a Patient with Atypical Ulcerative Colitis
- Long Noncoding RNA FBXL19-AS1-Mediated Ulcerative Colitis-Associated Intestinal Epithelial Barrier Defect