Investig Clin Urol.
2020 Mar;61(2):216-223. 10.4111/icu.2020.61.2.216.
Establishment of patient-derived three-dimensional organoid culture in renal cell carcinoma
- Affiliations
-
- 1Department of Urology, Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea. hanwk@yuhs.ac
- 2Department of Urology, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 3Department of Urology, Hanyang University College of Medicine, Seoul, Korea.
- 4Department of Urology, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 5Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2471078
- DOI: http://doi.org/10.4111/icu.2020.61.2.216
Abstract
- PURPOSE
Renal cell carcinoma is a heterogeneous kidney cancer, and over 403,000 cases were reported worldwide in 2018. Current methods for studying renal cell carcinoma are limited to two-dimensional (2D) culture of primary cell lines and patient-derived xenograft models. Numerous studies have suggested that 2D culture poorly represents the diversity, heterogeneity, and drug-resistance of primary tumors. The time and cost associated with patient-derived xenograft models poses a realistic barrier to their clinical utility. As a biomimetic model, patient-derived three-dimensional (3D) organoid culture can overcome these disadvantages and bridge the gap between in vitro cell culture and in vivo patient-derived xenograft models. Here, we establish a patient-derived 3D organoid culture system for clear cell renal cell carcinoma and demonstrate the biomimetic characteristics of our model with respect to both primary kidney cancer and conventional 2D culture.
MATERIALS AND METHODS
Normal renal tissues and tumor tissues were collected from patients with clear cell renal cell carcinoma. The dissociated cells were cultured as conventional 2D culture and 3D organoid culture. The biomimetic characteristic of the two cultures were compared.
RESULTS
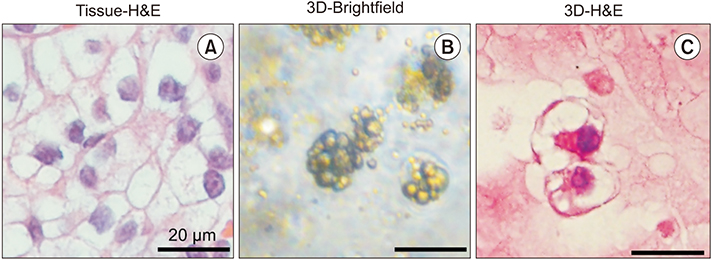
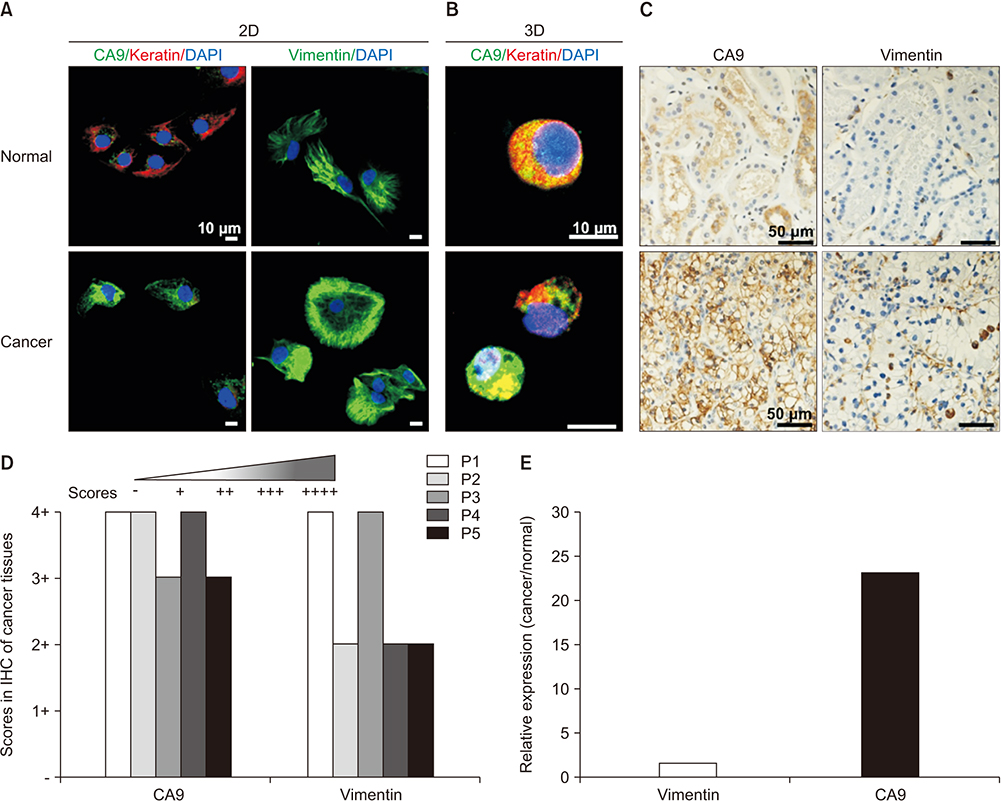
Compared with 2D culture, the 3D organoid cultures retained the characteristic lipid-rich, clear cell morphology of clear cell renal cell carcinoma. Carbonic anhydrase 9 and vimentin were validated as biomarkers of renal cell carcinoma. Expression of the two validated biomarkers was more enhanced in 3D organoid culture.
CONCLUSIONS
Patient-derived 3D organoid culture retains the characteristics of renal cell carcinoma with respect to morphology and biomarker expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Cairns P. Renal cell carcinoma. Cancer Biomark. 2010; 9:461–473.2. Choueiri TK, Motzer RJ. Systemic therapy for metastatic renalcell carcinoma. N Engl J Med. 2017; 376:354–366.
Article3. Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017; 7:462–477.
Article4. Sachs N, Clevers H. Organoid cultures for the analysis of cancer phenotypes. Curr Opin Genet Dev. 2014; 24:68–73.
Article5. Ben-David U, Ha G, Tseng YY, Greenwald NF, Oh C, Shih J, et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet. 2017; 49:1567–1575.
Article6. Mitra A, Mishra L, Li S. Technologies for deriving primary tumor cells for use in personalized cancer therapy. Trends Biotechnol. 2013; 31:347–354.
Article7. Sharma R, Greenhough S, Medine CN, Hay DC. Three-dimensional culture of human embryonic stem cell derived hepatic endoderm and its role in bioartificial liver construction. J Biomed Biotechnol. 2010; 2010:236147.
Article8. Malaney P, Nicosia SV, Davé V. One mouse, one patient paradigm: new avatars of personalized cancer therapy. Cancer Lett. 2014; 344:1–12.
Article9. Gao D, Chen Y. Organoid development in cancer genome discovery. Curr Opin Genet Dev. 2015; 30:42–48.
Article10. Grassi L, Alfonsi R, Francescangeli F, Signore M, De Angelis ML, Addario A, et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019; 10:201.
Article11. Artegiani B, Clevers H. Use and application of 3D-organoid technology. Hum Mol Genet. 2018; 27(R2):R99–R107.
Article12. Miyoshi T, Hiratsuka K, Saiz EG, Morizane R. Kidney organoids in translational medicine: disease modeling and regenerative medicine. Dev Dyn. 2019; 2015:03. 06. [Epub]. DOI: 10.1002/dvdy.22.
Article13. Morizane R, Bonventre JV. Kidney organoids: a translational journey. Trends Mol Med. 2017; 23:246–263.
Article14. Cantrell MA, Kuo CJ. Organoid modeling for cancer precision medicine. Genome Med. 2015; 7:32.
Article15. Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016; 18:246–254.
Article16. Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003; 349:366–381.
Article17. Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014; 159:176–187.
Article18. Takai A, Fako V, Dang H, Forgues M, Yu Z, Budhu A, et al. Three-dimensional organotypic culture models of human hepatocellular carcinoma. Sci Rep. 2016; 6:21174.
Article19. van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015; 161:933–945.
Article20. Jun DY, Kim SY, Na JC, Lee HH, Kim J, Yoon YE, et al. Tubular organotypic culture model of human kidney. PLoS One. 2018; 13:e0206447.
Article21. Prasad SR, Humphrey PA, Catena JR, Narra VR, Srigley JR, Cortez AD, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006; 26:1795–1806. discussion 806–10.
Article22. Ingels A, Hew M, Algaba F, de Boer OJ, van Moorselaar RJ, Horenblas S, et al. Vimentin over-expression and carbonic anhydrase IX under-expression are independent predictors of recurrence, specific and overall survival in non-metastatic clear-cell renal carcinoma: a validation study. World J Urol. 2017; 35:81–87.
Article23. Pickering LM, Larkin J. Kidney cancer: carbonic anhydrase IX in resected clear cell RCC. Nat Rev Urol. 2015; 12:309–310.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishment of an Organoid Culture Model Derived from Small Intestinal Epithelium of C57BL/6 Mice and Its Benefits over Tissues
- Increased SOX2 expression in three-dimensional sphere culture of dental pulp stem cells
- Sebaceous Carcinoma and Basal Cell Epithelioma Developed in Organoid Nevus
- Establishment of Three Dimensional In Vitro Laboratory Model in Squamous Cell Carcinoma of the Head and Neck: Spheroid Model and Raft Culture Model
- Gastric Organoid, a Promising Modeling for Gastric Stem Cell Homeostasis and Therapeutic Application