Investig Clin Urol.
2020 Mar;61(2):166-172. 10.4111/icu.2020.61.2.166.
BRCA1-associated protein 1 expression and prognostic role in prostate adenocarcinoma
- Affiliations
-
- 1Department of Pathology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea. chkap@korea.ac.kr
- 2Department of Urology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2471072
- DOI: http://doi.org/10.4111/icu.2020.61.2.166
Abstract
- PURPOSE
As prostate cancer (PCa) is the second most commonly diagnosed cancer worldwide, finding novel markers for prognosis is crucial. BRCA1-associated protein 1 (BAP-1), a nuclear-localized deubiquitinating enzyme, has been reported in several human cancers. However, its prognostic role in PCa remains unknown. Herein, we assessed the prognostic and clinicopathologic significance of BAP-1 in PCa.
MATERIALS AND METHODS
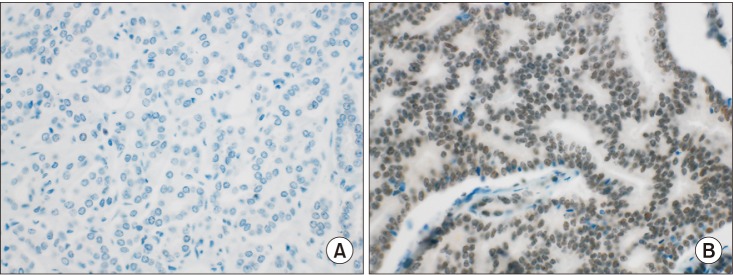
Seventy surgical specimens from radical prostatectomy cases were examined. Two cores per case were selected for construction of tissue microarrays (TMAs). After the exclusion of two cases because of tissue sparsity, BAP-1 immunohistochemical expression was evaluated in 68 cases of formalin-fixed, paraffin-embedded TMA tissue blocks. The immunohistochemical stain was scored according to proportion of nuclear staining: negative (<10% of tumor cells) or positive (≥10% of tumor cells).
RESULTS
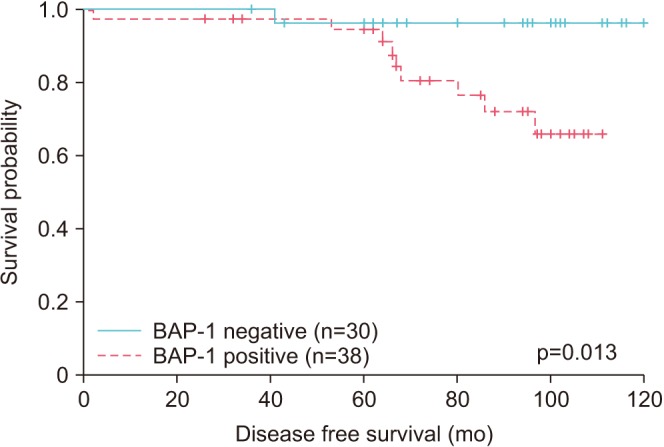
BAP-1 expression was negative in 30 cases (44.1%) and positive in 38 cases (55.9%). Positive BAP-1 expression was more common in pT3b disease than in pT2 (p=0.038). A high preoperative prostate-specific antigen level was correlated with BAP-1 expression (p=0.014). Age, lymphovascular invasion, perineural invasion, and grade group were not significantly correlated with BAP-1 expression. Patients with positive BAP-1 expression showed significantly shorter disease-free survival (p=0.013). Additionally, BAP-1 was an independent prognostic factor of PCa (p=0.035; hazard ratio, 9.277; 95% confidence interval, 1.165-73.892).
CONCLUSIONS
Our study findings showed an association of BAP-1 expression with poor PCa prognosis and suggest a potential role for BAP-1 as a prognostic biomarker for PCa.
Keyword
MeSH Terms
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.
Article2. Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998; 16:1097–1112. PMID: 9528852.
Article3. Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 2009; 69:111–119. PMID: 19117993.
Article4. Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008; 68:6953–6962. PMID: 18757409.
Article5. Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem. 2009; 284:34179–34188. PMID: 19815555.
Article6. Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet. 2016; 89:285–294. PMID: 26096145.7. Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant esothelioma. Nat Genet. 2011; 43:1022–1025. PMID: 21874000.8. Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d'Enghien C, et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet. 2013; 92:974–980. PMID: 23684012.
Article9. Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet. 2011; 43:1018–1021. PMID: 21874003.
Article10. Klebe S, Driml J, Nasu M, Pastorino S, Zangiabadi A, Henderson D, et al. BAP1 hereditary cancer predisposition syndrome: a case report and review of literature. Biomark Res. 2015; 3:14. PMID: 26140217.
Article11. Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011; 43:668–672. PMID: 21642991.
Article12. Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010; 330:1410–1413. PMID: 21051595.13. Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012; 44:751–759. PMID: 22683710.
Article14. Wang XY, Wang Z, Huang JB, Ren XD, Ye D, Zhu WW, et al. Tissue-specific significance of BAP1 gene mutation in prognostic prediction and molecular taxonomy among different types of cancer. Tumour Biol. 2017; 39:1010428317699111. PMID: 28618948.
Article15. Arzt L, Quehenberger F, Halbwedl I, Mairinger T, Popper HH. BAP1 protein is a progression factor in malignant pleural mesothelioma. Pathol Oncol Res. 2014; 20:145–151. PMID: 23963927.
Article16. Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H, et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. Am J Surg Pathol. 2012; 36:818–830. PMID: 22367297.
Article17. Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. Grading Committee. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016; 40:244–252. PMID: 26492179.18. Amin MB. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed. New York: Springer;2017.19. Freedland SJ, Sutter ME, Dorey F, Aronson WJ. Defining the ideal cutpoint for determining PSA recurrence after radical prostatectomy. Prostate-specific antigen. Urology. 2003; 61:365–369. PMID: 12597949.20. Shah AA, Bourne TD, Murali R. BAP1 protein loss by immunohistochemistry: a potentially useful tool for prognostic prediction in patients with uveal melanoma. Pathology. 2013; 45:651–656. PMID: 24247622.
Article21. Julien E, Herr W. Proteolytic processing is necessary to separate and ensure proper cell growth and cytokinesis functions of HCF-1. EMBO J. 2003; 22:2360–2369. PMID: 12743030.
Article22. Luchini C, Veronese N, Yachida S, Cheng L, Nottegar A, Stubbs B, et al. Different prognostic roles of tumor suppressor gene BAP1 in cancer: a systematic review with meta-analysis. Genes Chromosomes Cancer. 2016; 55:741–749. PMID: 27223342.23. Fukuda T, Tsuruga T, Kuroda T, Nishikawa H, Ohta T. Functional link between BRCA1 and BAP1 through histone H2A, heterochromatin and DNA damage response. Curr Cancer Drug Targets. 2016; 16:101–109. PMID: 26517537.
Article24. Omari A, Nastaly P, Balabas A, Dąbrowska M, Bielińska B, Huss S, et al. Somatic aberrations of BRCA1 gene are associated with progressive and stem cell-like phenotype of prostate cancer. bioRxiv. 2018; 271312.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The BRCA1 Expression in Placenta of Normal and Fetal Growth Restriction
- Significance of S100A2 and S100A4 Expression in the Progression of Prostate Adenocarcinoma
- Yes-Associated Protein Expression Is Correlated to the Differentiation of Prostate Adenocarcinoma
- Expression of BRCA1 Transcripts and Protein in Sporadic Ovarian Cancer
- The Expression of ERCC1, RRM1, and BRCA1 in Breast Cancer According to the Immunohistochemical Phenotypes