Korean J Physiol Pharmacol.
2020 Mar;24(2):129-135. 10.4196/kjpp.2020.24.2.129.
Connecting the dots between SHP2 and glutamate receptors
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea. yongseok7@snu.ac.kr
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea.
- 3Neuroscience Research Institute, Seoul National University College of Medicine, Seoul 03080, Korea.
- KMID: 2471034
- DOI: http://doi.org/10.4196/kjpp.2020.24.2.129
Abstract
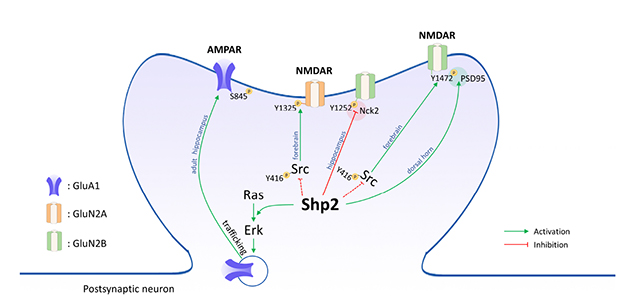
- SHP2 is an unusual protein phosphatase that functions as an activator for several signaling pathways, including the RAS pathway, while most other phosphatases suppress their downstream signaling cascades. The physiological and pathophysiological roles of SHP2 have been extensively studied in the field of cancer research. Mutations in the PTPN11 gene which encodes SHP2 are also highly associated with developmental disorders, such as Noonan syndrome (NS), and cognitive deficits including learning disabilities are common among NS patients. However, the molecular and cellular mechanism by which SHP2 is involved in cognitive functions is not well understood. Recent studies using SHP2 mutant mice or pharmacological inhibitors have shown that SHP2 plays critical role in learning and memory and synaptic plasticity. Here, we review the recent studies demonstrating that SHP2 is involved in synaptic plasticity, and learning and memory, by the regulation of the expression and/or function of glutamate receptors. We suggest that each cell type may have distinct paths connecting the dots between SHP2 and glutamate receptors, and these paths may also change with aging.
MeSH Terms
Figure
Reference
-
1. Jamieson CR, van der Burgt I, Brady AF, van Reen M, Elsawi MM, Hol F, Jeffery S, Patton MA, Mariman E. Mapping a gene for Noonan syndrome to the long arm of chromosome 12. Nat Genet. 1994; 8:357–360.
Article2. Ahmad S, Banville D, Zhao Z, Fischer EH, Shen SH. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci U S A. 1993; 90:2197–2201.
Article3. Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998; 92:441–450.
Article4. Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998; 6:249–254.
Article5. Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003; 28:284–293.
Article6. Tajan M, de Rocca Serra A, Valet P, Edouard T, Yart A. SHP2 sails from physiology to pathology. Eur J Med Genet. 2015; 58:509–525.
Article7. Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J Biol Chem. 2003; 278:41677–41684.
Article8. Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 2008; 20:453–459.
Article9. Shi ZQ, Yu DH, Park M, Marshall M, Feng GS. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000; 20:1526–1536.
Article10. Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006; 281:6785–6792.
Article11. Marin TM, Keith K, Davies B, Conner DA, Guha P, Kalaitzidis D, Wu X, Lauriol J, Wang B, Bauer M, Bronson R, Franchini KG, Neel BG, Kontaridis MI. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011; 121:1026–1043.
Article12. Bonetti M, Paardekooper Overman J, Tessadori F, Noël E, Bakkers J, den Hertog J. Noonan and LEOPARD syndrome Shp2 variants induce heart displacement defects in zebrafish. Development. 2014; 141:1961–1970.
Article13. Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, et al. Patient-specific induced pluripotent stemcell-derived models of LEOPARD syndrome. Nature. 2010; 465:808–812.
Article14. Edouard T, Montagner A, Dance M, Conte F, Yart A, Parfait B, Tauber M, Salles JP, Raynal P. How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms? Cell Mol Life Sci. 2007; 64:1585–1590.
Article15. Chan RJ, Johnson SA, Li Y, Yoder MC, Feng GS. A definitive role of Shp-2 tyrosine phosphatase in mediating embryonic stem cell differentiation and hematopoiesis. Blood. 2003; 102:2074–2080.
Article16. Ivins Zito C, Kontaridis MI, Fornaro M, Feng GS, Bennett AM. SHP-2 regulates the phosphatidylinositide 3'-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol. 2004; 199:227–236.
Article17. You M, Yu DH, Feng GS. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol. 1999; 19:2416–2424.
Article18. Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, Yang W, Pao LI, Gilliland DG, Epstein JA, Neel BG. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004; 10:849–857.
Article19. Lu H, Ash RT, He L, Kee SE, Wang W, Yu D, Hao S, Meng X, Ure K, Ito-Ishida A, Tang B, Sun Y, Ji D, Tang J, Arenkiel BR, Smirnakis SM, Zoghbi HY. Loss and gain of MeCP2 cause similar hippocampal circuit dysfunction that is rescued by deep brain stimulation in a rett syndrome mouse model. Neuron. 2016; 91:739–747.
Article20. Tartaglia M, Gelb BD. Noonan syndrome and related disorders: genetics and pathogenesis. Annu Rev Genomics Hum Genet. 2005; 6:45–68.
Article21. Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, Pierpont ME, Roberts AE, Robinson W, Takemoto CM, Noonan JA. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010; 126:746–759.
Article22. Adviento B, Corbin IL, Widjaja F, Desachy G, Enrique N, Rosser T, Risi S, Marco EJ, Hendren RL, Bearden CE, Rauen KA, Weiss LA. Autism traits in the RASopathies. J Med Genet. 2014; 51:10–20.
Article23. Lee DA, Portnoy S, Hill P, Gillberg C, Patton MA. Psychological profile of children with Noonan syndrome. Dev Med Child Neurol. 2005; 47:35–38.
Article24. Pierpont EI, Tworog-Dube E, Roberts AE. Learning and memory in children with Noonan syndrome. Am J Med Genet A. 2013; 161A:2250–2257.
Article25. Jindal GA, Goyal Y, Burdine RD, Rauen KA, Shvartsman SY. RASopathies: unraveling mechanisms with animal models. Dis Model Mech. 2015; 8:1167.
Article26. Bale TL, Abel T, Akil H, Carlezon WA Jr, Moghaddam B, Nestler EJ, Ressler KJ, Thompson SM. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019; 44:1349–1353.
Article27. Howe JR 6th, Bear MF, Golshani P, Klann E, Lipton SA, Mucke L, Sahin M, Silva AJ. The mouse as a model for neuropsychiatric drug development. Curr Biol. 2018; 28:R909–R914.
Article28. Pagani MR, Oishi K, Gelb BD, Zhong Y. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell. 2009; 139:186–198.
Article29. Tajan M, Paccoud R, Branka S, Edouard T, Yart A. The RASopathy family: consequences of germline activation of the RAS/MAPK pathway. Endocr Rev. 2018; 39:676–700.
Article30. Araki T, Chan G, Newbigging S, Morikawa L, Bronson RT, Neel BG. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc Natl Acad Sci U S A. 2009; 106:4736–4741.
Article31. Lee YS, Ehninger D, Zhou M, Oh JY, Kang M, Kwak C, Ryu HH, Butz D, Araki T, Cai Y, Balaji J, Sano Y, Nam CI, Kim HK, Kaang BK, Burger C, Neel BG, Silva AJ. Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome. Nat Neurosci. 2014; 17:1736–1743.
Article32. Levy AD, Xiao X, Shaw JE, Sudarsana Devi SP, Katrancha SM, Bennett AM, Greer CA, Howe JR, Machida K, Koleske AJ. Noonan syndrome-associated SHP2 dephosphorylates GluN2B to regulate NMDA receptor function. Cell Rep. 2018; 24:1523–1535.
Article33. Ryu HH, Kim T, Kim JW, Kang M, Park P, Kim YG, Kim H, Ha J, Choi JE, Lee J, Lim CS, Kim CH, Kim SJ, Silva AJ, Kaang BK, Lee YS. Excitatory neuron-specific SHP2-ERK signaling network regulates synaptic plasticity and memory. Sci Signal. 2019; 12:eaau5755.
Article34. Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994; 4:389–399.
Article35. Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science. 2011; 334:623–628.
Article36. Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009; 10:126–140.
Article37. Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008; 9:65–75.
Article38. Kusakari S, Saitow F, Ago Y, Shibasaki K, Sato-Hashimoto M, Matsuzaki Y, Kotani T, Murata Y, Hirai H, Matsuda T, Suzuki H, Matozaki T, Ohnishi H. Shp2 in forebrain neurons regulates synaptic plasticity, locomotion, and memory formation in mice. Mol Cell Biol. 2015; 35:1557–1572.
Article39. Yan X, Zhang B, Lu W, Peng L, Yang Q, Cao W, Lin S, Yu W, Li X, Ke Y, Li S, Yang W, Luo J. Increased Src Family kinase activity disrupts excitatory synaptic transmission and impairs remote fear memory in forebrain Shp2-Deficient mice. Mol Neurobiol. 2017; 54:7235–7250.
Article40. Fornaro M, Burch PM, Yang W, Zhang L, Hamilton CE, Kim JH, Neel BG, Bennett AM. SHP-2 activates signaling of the nuclear factor of activated T cells to promote skeletal muscle growth. J Cell Biol. 2006; 175:87–97.
Article41. Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004; 101:16064–16069.
Article42. Lüscher C, Nicoll RA, Malenka RC, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000; 3:545–550.
Article43. Butcher SP, Davis S, Morris RG. A dose-related impairment of spatial learning by the NMDA receptor antagonist, 2-amino-5-phosphonovalerate (AP5). Eur Neuropsychopharmacol. 1990; 1:15–20.
Article44. Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992; 12:21–34.45. Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996; 87:1327–1338.
Article46. Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000; 3:661–669.
Article47. Lin SY, Wu K, Len GW, Xu JL, Levine ES, Suen PC, Mount HT, Black IB. Brain-derived neurotrophic factor enhances association of protein tyrosine phosphatase PTP1D with the NMDA receptor subunit NR2B in the cortical postsynaptic density. Brain Res Mol Brain Res. 1999; 70:18–25.
Article48. Peng HY, Chen GD, Lai CY, Hsieh MC, Lin TB. Spinal SIRPα1-SHP2 interaction regulates spinal nerve ligation-induced neuropathic pain via PSD-95-dependent NR2B activation in rats. Pain. 2012; 153:1042–1053.
Article49. Ding X, Cai J, Li S, Liu XD, Wan Y, Xing GG. BDNF contributes to the development of neuropathic pain by induction of spinal long-term potentiation via SHP2 associated GluN2B-containing NMDA receptors activation in rats with spinal nerve ligation. Neurobiol Dis. 2015; 73:428–451.
Article50. Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002; 25:571–577.
Article51. Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007; 54:859–871.
Article52. Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013; 80:704–717.
Article53. Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018; 100:314–329.
Article54. Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000; 405:955–959.
Article55. Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998; 21:1163–1175.
Article56. Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaMKII during long-term potentiation. Science. 1997; 276:2042–2045.
Article57. Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993; 261:1051–1055.
Article58. Zhang B, Du YL, Lu W, Yan XY, Yang Q, Yang W, Luo JH. Increased activity of Src homology 2 domain containing phosphotyrosine phosphatase 2 (Shp2) regulates activity-dependent AMPA receptor trafficking. J Biol Chem. 2016; 291:18856–18866.
Article59. Zhang B, Lu W. Src homology 2 domain-containing phosphotyrosine phosphatase 2 (Shp2) controls surface GluA1 protein in synaptic homeostasis. J Biol Chem. 2017; 292:15481–15488.
Article60. Oh JY, Rhee S, Silva AJ, Lee YS, Kim HK. Noonan syndrome-associated SHP2 mutation differentially modulates the expression of postsynaptic receptors according to developmental maturation. Neurosci Lett. 2017; 649:41–47.
Article61. Stornetta RL, Zhu JJ. Ras and Rap signaling in synaptic plasticity and mental disorders. Neuroscientist. 2011; 17:54–78.
Article62. Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002; 110:443–455.
Article63. Ohnishi H, Murata Y, Okazawa H, Matozaki T. Src family kinases: modulators of neurotransmitter receptor function and behavior. Trends Neurosci. 2011; 34:629–637.
Article64. Zhang SQ, Yang W, Kontaridis MI, Bivona TG, Wen G, Araki T, Luo J, Thompson JA, Schraven BL, Philips MR, Neel BG. Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment. Mol Cell. 2004; 13:341–355.
Article65. Suzuki T, Matozaki T, Mizoguchi A, Kasuga M. Localization and subcellular distribution of SH-PTP2, a protein-tyrosine phosphatase with Src homology-2 domains, in rat brain. Biochem Biophys Res Commun. 1995; 211:950–959.66. Servidei T, Bhide PG, Huang Z, Moskowitz MA, Harsh G, Reeves SA. The protein tyrosine phosphatase SHP-2 is expressed in glial and neuronal progenitor cells, postmitotic neurons and reactive astrocytes. Neuroscience. 1998; 82:529–543.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TET3-mediated DNA demethylation modification activates SHP2 expression to promote endometrial cancer progression through the EGFR/ERK pathway
- Anethesia and Glutamate Receptors
- The ontogeny of excitatory amino acid receptors in the rat brain quantitative autoradiographic study: I. N-methyl-D-aspartate receptors
- Newer Antipsychotics:Serotonin and Glutamate Receptor Related Drugs
- Effect of Brain-derived Neurotrophic Factor on Excitatory Synaptic Transmission in Hippocampal Neurons