Ann Lab Med.
2020 Jul;40(4):285-296. 10.3343/alm.2020.40.4.285.
Cortisol Measurements in Cushing's Syndrome: Immunoassay or Mass Spectrometry?
- Affiliations
-
- 1Department of Biochemistry and Molecular Genetics, Hospital ClÃnic, Barcelona, Spain. casals@clinic.cat
- 2Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain.
- 3Centrode Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), ISCIII, Madrid, Spain.
- 4Department of Endocrinology and Nutrition, Hospital ClÃnic, Barcelona, Spain.
- 5Department of Medicine, Faculty of Medicine and Health Sciences, University of Barcelona, Barcelona, Spain.
- KMID: 2470324
- DOI: http://doi.org/10.3343/alm.2020.40.4.285
Abstract
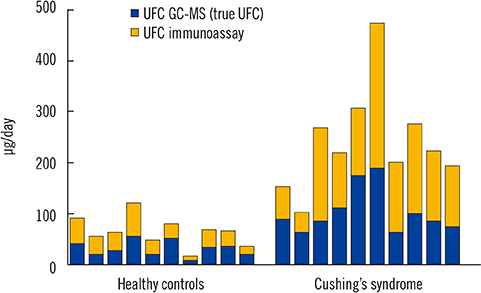
- Determination of cortisol levels in the urine (24 hours urine free cortisol), saliva (late-night), or serum (total cortisol after dexamethasone suppression) is recommended to screen for Cushing's syndrome (CS). This review focuses on the differences between the frequently used cortisol-antibody immunoassay-based methods and the highly specific mass-spectrometry-based methods that are progressively being employed in clinical laboratories for CS screening. The particular characteristics of cortisol metabolism and the lack of specificity of the immunoassays cause marked differences between both methods that are in turn highly dependent on the biological matrix, in which the cortisol is measured. Understanding the origin of these differences is essential for the interpretation of these results. Although cross-reactivity with endogenous steroids leads to grossly inaccurate results of immunoassay measurements of cortisol in the saliva and urine, preliminary evidence suggests that the clinical sensitivity of CS screening using immunoassays may be similar to CS screening using mass spectrometry. However, mass spectrometry offers more accurate results and considerably reduced variation across laboratories, while avoiding false-positive results. Moreover, mass spectrometry can overcome some common diagnostic challenges, such as identification of exogenous corticosteroids or simultaneous assessment of appropriate dexamethasone levels in suppression tests. Further, comprehensive mass spectrometry-based profiling of several steroid metabolites may be useful for discriminating among different subtypes of CS. Finally, this review discusses the main preanalytical factors that could cause variations in cortisol measurements and their influence on the reliability of the results.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Second-Line Tests in the Diagnosis of Adrenocorticotropic Hormone-Dependent Hypercortisolism
Silvia Pinelli, Mattia Barbot, Carla Scaroni, Filippo Ceccato
Ann Lab Med. 2021;41(6):521-531. doi: 10.3343/alm.2021.41.6.521.Clinical and Technical Aspects in Free Cortisol Measurement
Man Ho Choi
Endocrinol Metab. 2022;37(4):599-607. doi: 10.3803/EnM.2022.1549.
Reference
-
1. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015; 386:913–927.
Article2. Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing's disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011; 96:632–642.
Article3. Neary NM, Booker OJ, Abel BS, Matta JR, Muldoon N, Sinaii N, et al. Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab. 2013; 98:2045–2052.
Article4. Loriaux DL. Diagnosis and differential diagnosis of Cushing's syndrome. N Engl J Med. 2017; 376:1451–1459.
Article5. Pappachan JM, Hariman C, Edavalath M, Waldron J, Hanna FW. Cushing's syndrome: a practical approach to diagnosis and differential diagnoses. J Clin Pathol. 2017; 70:350–359.
Article6. Chabre O. The difficulties of pseudo-Cushing's syndrome (or ‘non-neoplastic hypercortisolism’). Ann Endocrinol (Paris). 2018; 79:138–145.
Article7. Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011; 96:1533–1540.
Article8. Chiodini I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011; 96:1223–1236.9. Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing's syndrome: a clinical challenge. Eur J Endocrinol. 2007; 157:245–254.
Article10. Manenschijn L, Koper JW, van den Akker EL, de Heide LJ, Geerdink EA, de Jong FH, et al. A novel tool in the diagnosis and follow-up of (cyclic) Cushing's syndrome: measurement of long-term cortisol in scalp hair. J Clin Endocrinol Metab. 2012; 97:E1836–E1843.
Article11. Hodes A, Meyer J, Lodish MB, Stratakis CA, Zilbermint M. Mini-review of hair cortisol concentration for evaluation of Cushing syndrome. Expert Rev Endocrinol Metab. 2018; 13:225–231.
Article12. Greff MJE, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, van Uum SHM. Hair cortisol analysis: an update on methodological considerations and clinical applications. Clin Biochem. 2019; 63:1–9.
Article13. Juszczak A, Sulentic P, Grossman A. Cushing's syndrome. In : Feingold KR, Anawalt B, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.;2000–2017.14. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008; 93:1526–1540.
Article15. Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2003; 88:5593–5602.
Article16. Barbot M, Trementino L, Zilio M, Ceccato F, Albiger N, Daniele A, et al. Second-line tests in the differential diagnosis of ACTH-dependent Cushing's syndrome. Pituitary. 2016; 19:488–495.
Article17. Aranda G, Enseñat J, Mora M, Puig-Domingo M, Martínez de Osaba MJ, Casals G, et al. Long-term remission and recurrence rate in a cohort of Cushing's disease: the need for long-term follow-up. Pituitary. 2015; 18:142–149.
Article18. Yeo KT, Babic N, Hannoush ZC, Weiss RE. Endocrine testing protocols: hypothalamic pituitary adrenal axis. In : Feingold KR, Anawalt B, editors. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.;2000–2017.19. Deutschbein T, Broecker-Preuss M, Hartmann MF, Althoff R, Wudy SA, Mann K, et al. Measurement of urinary free cortisol by current immunoassays: need for sex-dependent reference ranges to define hypercortisolism. Horm Metab Res. 2011; 43:714–719.
Article20. Miki K, Sudo A. Effect of urine pH, storage time, and temperature on stability of catecholamines, cortisol, and creatinine. Clin Chem. 1998; 44:1759–1762.
Article21. Hansen AM, Garde AH, Skovgaard LT, Christensen JM. Seasonal and biological variation of urinary epinephrine, norepinephrine, and cortisol in healthy women. Clin Chim Acta. 2001; 309:25–35.
Article22. Pecori Giraldi F, Ambrogio AG. Variability in laboratory parameters used for management of Cushing's syndrome. Endocrine. 2015; 50:580–589.
Article23. Chan KC, Lit LC, Law EL, Tai MH, Yung CU, Chan MH, et al. Diminished urinary free cortisol excretion in patients with moderate and severe renal impairment. Clin Chem. 2004; 50:757–759.
Article24. Turpeinen U, Hämäläinen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 2013; 27:795–801.
Article25. Wood L, Ducroq DH, Fraser HL, Gillingwater S, Evans C, Pickett AJ, et al. Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography-mass spectrometry and two commercial immunoassays. Ann Clin Biochem. 2008; 45:380–388.
Article26. Aranda G, Careaga M, Hanzu FA, Patrascioiu I, Ríos P, Mora M, et al. Accuracy of immunoassay and mass spectrometry urinary free cortisol in the diagnosis of Cushing's syndrome. Pituitary. 2016; 19:496–502.
Article27. McCann SJ, Gillingwater S, Keevil BG. Measurement of urinary free cortisol using liquid chromatography-tandem mass spectrometry: comparison with the urine adapted ACS:180 serum cortisol chemiluminescent immunoassay and development of a new reference range. Ann Clin Biochem. 2005; 42:112–118.
Article28. Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, Camozzi V, et al. Screening tests for Cushing's syndrome: urinary free cortisol role measured by LC-MS/MS. J Clin Endocrinol Metab. 2015; 100:3856–3861.
Article29. Ceccato F, Antonelli G, Barbot M, Zilio M, Mazzai L, Gatti R, et al. The diagnostic performance of urinary free cortisol is better than the cortisol: cortisone ratio in detecting de novo Cushing's syndrome: the use of a LC-MS/MS method in routine clinical practice. Eur J Endocrinol. 2014; 171:1–7.30. Murphy BE. How much “UFC” is really cortisol? Clin Chem. 2000; 46:793–794.
Article31. Raff H, Auchus RJ, Findling JW, Nieman LK. Urine free cortisol in the diagnosis of Cushing's syndrome: is it worth doing and, if so, how? J Clin Endocrinol Metab. 2015; 100:395–397.
Article32. Oßwald A, Wang R, Beuschlein F, Hartmann MF, Wudy SA, Bidlingmaier M, et al. Performance of LC-MS/MS and immunoassay based 24-h urine free cortisol in the diagnosis of Cushing's syndrome. J Steroid Biochem Mol Biol. 2019; 190:193–197.
Article33. El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva-are our assays good enough? Ann Clin Biochem. 2017; 54:308–322.34. Roberts RF, Roberts WL. Performance characteristics of five automated serum cortisol immunoassays. Clin Biochem. 2004; 37:489–493.
Article35. Fink RS, Pierre LN, Daley-Yates PT, Richards DH, Gibson A, Honour JW. Hypothalamic-pituitary-adrenal axis function after inhaled corticosteroids: unreliability of urinary free cortisol estimation. J Clin Endocrinol Metab. 2002; 87:4541–4546.
Article36. Thynne T, White GH, Burt MG. Factitious Cushing's syndrome masquerading as Cushing's disease. Clin Endocrinol (Oxf). 2014; 80:328–332.
Article37. Djedovic NK, Rainbow SJ. Detection of synthetic glucocorticoids by liquid chromatography-tandem mass spectrometry in patients being investigated for Cushing's syndrome. Ann Clin Biochem. 2011; 48:542–549.
Article38. Taylor RL, Grebe SK, Singh RJ. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem. 2004; 50:2345–2352.
Article39. Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH. Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol. 2010; 121:496–504.
Article40. Marcos J, Renau N, Casals G, Segura J, Ventura R, Pozo OJ. Investigation of endogenous corticosteroids profiles in human urine based on liquid chromatography tandem mass spectrometry. Anal Chim Acta. 2014; 812:92–104.
Article41. Casals G, Marcos J, Pozo OJ, Alcaraz J, Martínez de Osaba MJ, Jiménez W. Microwave-assisted derivatization: application to steroid profiling by gas chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014; 960:8–13.
Article42. Brossaud J, Ducint D, Corcuff JB. Urinary glucocorticoid metabolites: biomarkers to classify adrenal incidentalomas? Clin Endocrinol (Oxf). 2016; 84:236–243.
Article43. Ceccato F, Trementino L, Barbot M, Antonelli G, Plebani M, Denaro L, et al. Diagnostic accuracy of increased urinary cortisol/cortisone ratio to differentiate ACTH-dependent Cushing's syndrome. Clin Endocrinol (Oxf). 2017; 87:500–507.
Article44. Kulle AE, Welzel M, Holterhus PM, Riepe FG. Principles and clinical applications of liquid chromatography-tandem mass spectrometry for the determination of adrenal and gonadal steroid hormones. J Endocrinol Invest. 2011; 34:702–708.45. Brossaud J, Leban M, Corcuff JB, Boux de Casson F, Leloupp AG, Masson D, et al. LC-MSMS assays of urinary cortisol, a comparison between four in-house assays. Clin Chem Lab Med. 2018; 56:1109–1116.
Article46. Carroll T, Raff H, Findling JW. Late-night salivary cortisol for the diagnosis of Cushing syndrome: a meta-analysis. Endocr Pract. 2009; 15:335–342.
Article47. Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, et al. Accuracy of diagnostic tests for Cushing's syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab. 2008; 93:1553–1562.
Article48. Raff H, Phillips JM. Bedtime salivary cortisol and cortisone by LC-MS/MS in healthy adult subjects: evaluation of sampling time. J Endocr Soc. 2019; 3:1631–1640.
Article49. Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab. 2007; 92:3102–3107.
Article50. Casals G, Foj L, de Osaba MJ. Day-to-day variation of late-night salivary cortisol in healthy voluntaries. Clin Biochem. 2011; 44:665–668.
Article51. Sandouk Z, Johnston P, Bunch D, Wang S, Bena J, Hamrahian A, et al. Variability of late-night salivary cortisol in Cushing disease: A prospective study. J Clin Endocrinol Metab. 2018; 103:983–990.
Article52. Coelli S, Farias CB, Soares AA, Crescente GM, Hirakata VN, Souza LB, et al. Influence of age, gender and body mass index on late-night salivary cortisol in healthy adults. Clin Chem Lab Med. 2017; 55:1954–1961.
Article53. Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012-laboratory techniques and clinical indications. Clin Endocrinol (Oxf). 2012; 77:645–651.54. Calvi JL, Chen FR, Benson VB, Brindle E, Bristow M, De A, et al. Measurement of cortisol in saliva: a comparison of measurement error within and between international academic-research laboratories. BMC Res Notes. 2017; 10:479.
Article55. Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, et al. Salivary cortisol as a diagnostic tool for Cushing's syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol. 2012; 166:613–618.
Article56. Vogeser M, Durner J, Seliger E, Auernhammer C. Measurement of late-night salivary cortisol with an automated immunoassay system. Clin Chem Lab Med. 2006; 44:1441–1445.
Article57. Ceccato F, Marcelli G, Martino M, Concettoni C, Brugia M, Trementino L, et al. The diagnostic accuracy of increased late night salivary cortisol for Cushing's syndrome: a real-life prospective study. J Endocrinol Invest. 2019; 42:327–335.
Article58. Monaghan PJ, Keevil BG, Trainer PJ. The use of mass spectrometry to improve the diagnosis and the management of the HPA axis. Rev Endocr Metab Disord. 2013; 14:143–157.
Article59. Beko G, Varga I, Glaz E, Sereg M, Feldman K, Toth M, et al. Cutoff values of midnight salivary cortisol for the diagnosis of overt hypercortisolism are highly influenced by methods. Clin Chim Acta. 2010; 411:364–367.
Article60. Raff H. Cushing's syndrome: diagnosis and surveillance using salivary cortisol. Pituitary. 2012; 15:64–70.
Article61. Mészáros K, Karvaly G, Márta Z, Magda B, Tőke J, Szücs N, et al. Diagnostic performance of a newly developed salivary cortisol and cortisone measurement using an LC-MS/MS method with simple and rapid sample preparation. J Endocrinol Invest. 2018; 41:315–323.
Article62. Kline GA, Buse JD, Van Der Gugten JG, Holmes DT, Chin AC, Sadrzadeh SMH. Factitious ACTH-dependent, apparent hypercortisolism: the problem with late-night salivary cortisol measurements collected at home. Clin Endocrinol (Oxf). 2017; 87:882–885.
Article63. Raff H, Singh RJ. Measurement of late-night salivary cortisol and cortisone by LC-MS/MS to assess preanalytical sample contamination with topical hydrocortisone. Clin Chem. 2012; 58:947–948.
Article64. Israelsson M, Brattsand R, Brattsand G. 20α- and 20β-dihydrocortisone may interfere in LC-MS/MS determination of cortisol in saliva and urine. Ann Clin Biochem. 2018; 55:341–347.
Article65. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016; 175:G1–G34.
Article66. Nieman LK. Recent updates on the diagnosis and management of Cushing's syndrome. Endocrinol Metab (Seoul). 2018; 33:139–146.
Article67. Vastbinder M, Kuindersma M, Mulder AH, Schuijt MP, Mudde AH. The influence of oral contraceptives on overnight 1 mg dexamethasone suppression test. Neth J Med. 2016; 74:158–161.68. Meikle AW. Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clin Endocrinol (Oxf). 1982; 16:401–408.
Article69. Hempen C, Elfering S, Mulder AH, van den Bergh FA, Maatman RG. Dexamethasone suppression test: development of a method for simultaneous determination of cortisol and dexamethasone in human plasma by liquid chromatography/tandem mass spectrometry. Ann Clin Biochem. 2012; 49:170–176.
Article70. Ueland GÅ, Methlie P, Kellmann R, Bjørgaas M, Åsvold BO, Thorstensen K, et al. Simultaneous assay of cortisol and dexamethasone improved diagnostic accuracy of the dexamethasone suppression test. Eur J Endocrinol. 2017; 176:705–713.
Article71. Brixey-McCann R, Tennant SM, Geen J, Armston A, Barth JH, Keevil B, et al. Effect of cortisol assay bias on the overnight dexamethasone suppression test: implications for the investigation of Cushing's syndrome. Endocrine Abstracts. 2015; 38:22.
Article72. Monaghan PJ, Owen LJ, Trainer PJ, Brabant G, Keevil BG, Darby D. Comparison of serum cortisol measurement by immunoassay and liquid chromatography-tandem mass spectrometry in patients receiving the 11β-hydroxylase inhibitor metyrapone. Ann Clin Biochem. 2011; 48:441–446.
Article73. El-Farhan N, Pickett A, Ducroq D, Bailey C, Mitchem K, Morgan N, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol (Oxf). 2013; 78:673–680.
Article74. Masjkur J, Gruber M, Peitzsch M, Kaden D, Di Dalmazi G, Bidlingmaier M, et al. Plasma steroid profiles in subclinical compared with overt adrenal Cushing syndrome. J Clin Endocrinol Metab. 2019; 104:4331–4340.
Article75. Eisenhofer G, Masjkur J, Peitzsch M, Di Dalmazi G, Bidlingmaier M, Grüber M, et al. Plasma steroid metabolome profiling for diagnosis and subtyping patients with Cushing syndrome. Clin Chem. 2018; 64:586–596.
Article76. Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing's syndrome. J Clin Endocrinol Metab. 1998; 83:2681–2686.
Article77. Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing's syndrome. J Clin Endocrinol Metab. 2002; 87:4515–4521.
Article78. Putignano P, Toja P, Dubini A, Pecori Giraldi F, Corsello SM, Cavagnini F. Midnight salivary cortisol versus urinary free and midnight serum cortisol as screening tests for Cushing's syndrome. J Clin Endocrinol Metab. 2003; 88:4153–4157.
Article79. Yaneva M, Mosnier-Pudar H, Dugué MA, Grabar S, Fulla Y, Bertagna X. Midnight salivary cortisol for the initial diagnosis of Cushing's syndrome of various causes. J Clin Endocrinol Metab. 2004; 89:3345–3351.
Article80. Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Müller B. Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab. 2005; 90:5730–5736.
Article81. Bukan AP, Dere HB, Jadhav SS, Kasaliwal RR, Budyal SR, Shivane VK, et al. The performance and reproducibility of late-night salivary cortisol estimation by enzyme immunoassay for screening Cushing disease. Endocr Pract. 2015; 21:158–164.
Article82. Findling JW, Fleseriu M, Newell-Price J, Petersenn S, Pivonello R, Kandra A, et al. Late-night salivary cortisol may be valuable for assessing treatment response in patients with Cushing's disease: 12-month, Phase III pasireotide study. Endocrine. 2016; 54:516–523.
Article83. Ambroziak U, Kondracka A, Bartoszewicz Z, Krasnodębska-Kiljańska M, Bednarczuk T. The morning and late-night salivary cortisol ranges for healthy women may be used in pregnancy. Clin Endocrinol (Oxf). 2015; 83:774–778.
Article84. Lages AS, Frade JG, Oliveira D, Paiva I, Oliveira P, Rebelo-Marques A, et al. Late-night salivary cortisol: cut-off definition and diagnostic accuracy for Cushing's syndrome in a Portuguese population. Acta Med Port. 2019; 32:381–387.
Article85. Erickson D, Singh RJ, Sathananthan A, Vella A, Bryant SC. Late-night salivary cortisol for diagnosis of Cushing's syndrome by liquid chromatography/tandem mass spectrometry assay. Clin Endocrinol (Oxf). 2012; 76:467–472.
Article86. Palmieri S, Morelli V, Polledri E, Fustinoni S, Mercadante R, Olgiati L, et al. The role of salivary cortisol measured by liquid chromatography-tandem mass spectrometry in the diagnosis of subclinical hypercortisolism. Eur J Endocrinol. 2013; 168:289–296.
Article87. Antonelli G, Ceccato F, Artusi C, Marinova M, Plebani M. Salivary cortisol and cortisone by LC-MS/MS: validation, reference intervals and diagnostic accuracy in Cushing's syndrome. Clin Chim Acta. 2015; 451:247–251.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cushing's Syndrome
- Clinical and Technical Aspects in Free Cortisol Measurement
- Comparison of Direct and Extraction Immunoassay Methods With Liquid Chromatography-Tandem Mass Spectrometry Measurement of Urinary Free Cortisol for the Diagnosis of Cushing’s Syndrome
- Recent Updates on the Diagnosis and Management of Cushing's Syndrome
- A Sensitive and Specific Liquid ChromatographyTandem Mass Spectrometry Assay for Simultaneous Quantification of Salivary Melatonin and Cortisol: Development and Comparison With Immunoassays