Allergy Asthma Respir Dis.
2020 Jan;8(1):30-35. 10.4168/aard.2020.8.1.30.
Analysis of individual case safety reports of drug-induced anaphylaxis to the Korea Adverse Event Reporting System
- Affiliations
-
- 1Drug Safety Monitoring Center, Seoul National University Hospital, Seoul, Korea. helenmed@snu.ac.kr
- 2College of Pharmacy and Research Institute of Pharmaceutical Sciences, Seoul National University, Seoul, Korea.
- 3Institute of Allergy and Clinical Immunology, Seoul National University Medical Research Center, Seoul, Korea.
- 4Division of Allergy and Clinical Immunology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2469116
- DOI: http://doi.org/10.4168/aard.2020.8.1.30
Abstract
- PURPOSE
To identify causative agents of the drug-induced anaphylaxis (DIA) by using the Korea Institute of Drug Safety & Risk Management-Korea Adverse Event Reporting System (KIDS-KAERS) database (Ministry of Food and Drug Safety) in Korea and to check their labeling information regarding anaphylaxis.
METHODS
Among Individual Case Safety Reports from January, 2008 to December 2017, cases of DIA were analyzed for demographics, causative agents and fatal cases resulting in death. The domestic drug labeling, Micromedex and U.S. Food and Drug Administration (FDA) drug package insert, were reviewed to check if the labeling information on suspected causative agents contains anaphylaxis.
RESULTS
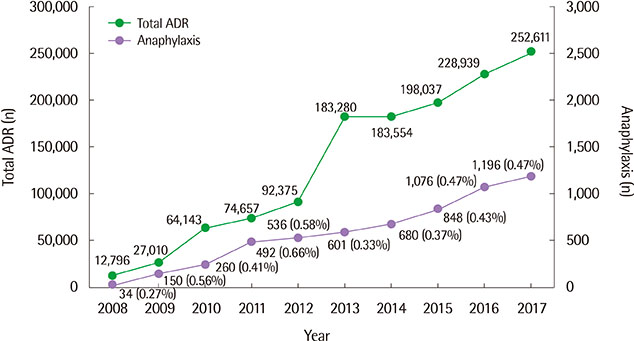
A total of 4,700 cases of DIA were analyzed. The mean age was 49.85±18.32 years, and 2,642 patients (56.2%) were females. Among 8,664 drugs reported as causative agents, antibiotics (27.4%) accounted for the largest portion. There were 18 fatal cases: antibiotics (7 cases), antineoplastic agents (4 cases) were the major causative drugs for the mortality cases. Of 513 drugs reported as suspected causative agents, 103 (20.1%) did not list anaphylaxis as an adverse effect on domestic drug labeling and 16 (3.1%) did not reflect anaphylaxis in any of 3 adverse drug information.
CONCLUSION
Analysis of 10-year data showed that antibiotics were the main cause of DIA and the mortality rate was 0.7%. In 3.1% of suspected drugs, there was no description of anaphylaxis in any of the drug labeling.
MeSH Terms
Figure
Cited by 1 articles
-
Drug-induced anaphylaxis: Analysis of the Pharmacovigilance Database
Sujeong Kim, Han-Ki Park
Allergy Asthma Respir Dis. 2020;8(1):1-2. doi: 10.4168/aard.2020.8.1.1.
Reference
-
1. Bochner BS, Lichtenstein LM. Anaphylaxis. N Engl J Med. 1991; 324:1785–1790.
Article2. Wood RA, Camargo CA Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014; 133:461–467.
Article3. Jeong K, Lee JD, Kang DR, Lee S. A population-based epidemiological study of anaphylaxis using national big data in Korea: trends in age-specific prevalence and epinephrine use in 2010–2014. Allergy Asthma Clin Immunol. 2018; 14:31.
Article4. Zhao Y, Sun S, Li X, Ma X, Tang H, Sun L, et al. Drug-induced anaphylaxis in China: a 10 year retrospective analysis of the Beijing Pharmacovigilance Database. Int J Clin Pharm. 2018; 40:1349–1358.
Article5. Ye YM, Kim MK, Kang HR, Kim TB, Sohn SW, Koh YI, et al. Predictors of the severity and serious outcomes of anaphylaxis in korean adults: a multicenter retrospective case study. Allergy Asthma Immunol Res. 2015; 7:22–29.
Article6. Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014; 134:1318–1328.
Article7. Yang MS, Lee SH, Kim TW, Kwon JW, Lee SM, Kim SH, et al. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008; 100:31–36.
Article8. Moon IJ, Park HJ, Kim SR, Koh BS, Leem DW, Park KH, et al. Drug-induced anaphylaxis in a single Korean tertiary hospital. Korean J Med. 2015; 88:281–287.
Article9. Carneiro-Leão L, Barzylovych V, Cernadas J. Pemetrexed anaphylaxis-an unusual suspect. J Allergy Clin Immunol Pract. 2019; 7:320–321.
Article10. Yoo HS, Yang EM, Kim MA, Hwang SH, Shin YS, Ye YM, et al. A case of codeine induced anaphylaxis via oral route. Allergy Asthma Immunol Res. 2014; 6:95–97.
Article11. Donado CD, Díez EM. Successful desensitization for hydroxychloroquine anaphylaxis. J Rheumatol. 2010; 37:1975–1976.
Article12. Faria E, Rodrigues-Cernadas J, Gaspar A, Botelho C, Castro E, Lopes A, et al. Drug-induced anaphylaxis survey in Portuguese Allergy Departments. J Investig Allergol Clin Immunol. 2014; 24:40–48.13. Korea Institute of Drug Safety & Risk Management. 2018 Drug safety information report. Anyang (Korea): Korea Institute of Drug Safety & Risk Management;2018.14. Ku JH, Kang PS, Cho CK, Jung SM, Lim YS, Kim SH, et al. Anaphylaxis induced by intravenous ranitidine injection: 2 case reports. Korean J Crit Care Med. 2010; 25:253–256.
Article15. Chopra D, Arora P, Khan S, Dwivedi S. Anaphylaxis following intravenous ranitidine: a rare adverse reaction of a common drug. Indian J Pharmacol. 2014; 46:234–236.
Article16. Park KH, Pai J, Song DG, Sim DW, Park HJ, Lee JH, et al. Ranitidine-induced anaphylaxis: clinical features, cross-reactivity, and skin testing. Clin Exp Allergy. 2016; 46:631–639.
Article17. Kim H, Fischer D. Anaphylaxis. Allergy Asthma Clin Immunol. 2011; 7 Suppl 1:S6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Change of the Etiology of Drug Induced Anaphylaxis in a Korean Tertiary Care Hospital
- Signal Detection of Adverse Event of Metoclopramide in Korea Adverse Event Reporting System (KAERS)
- Identifying the Patterns of Adverse Drug Responses of Cetuximab
- Pediatric Medication Error Reports in Korea Adverse Event Reporting System Database, 1989-2012: Comparing with Adult Reports
- Signal Detection and Safety Information Generation of Aripiprazole in Spontaneous Adverse Event Reports Database