J Korean Med Sci.
2015 Apr;30(4):371-377. 10.3346/jkms.2015.30.4.371.
Pediatric Medication Error Reports in Korea Adverse Event Reporting System Database, 1989-2012: Comparing with Adult Reports
- Affiliations
-
- 1Korea Institute of Drug Safety & Risk Management, Seoul, Korea. bjpark@drugsafe.or.kr
- 2Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2164443
- DOI: http://doi.org/10.3346/jkms.2015.30.4.371
Abstract
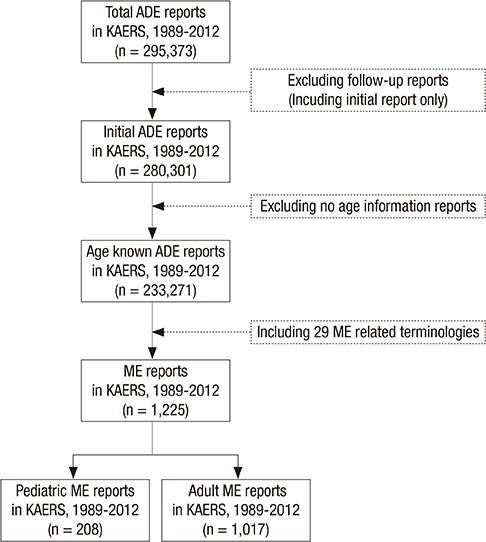
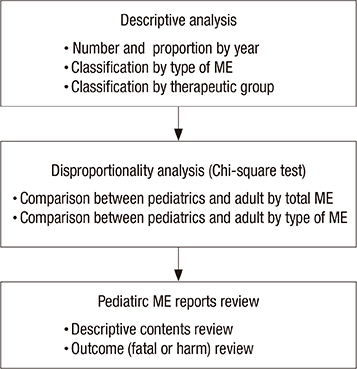
- Children have dynamic process of maturation and substantial changes in growth and development which eventually make the drug safety profiles different from adults. Medication errors (MEs) in pediatrics are reported to occur three times more likely than adults. The aims of this study were to identify the characteristics of pediatric MEs in Korea at national level and help raise awareness of risks from the MEs in pediatrics. We conducted a descriptive analysis with the pediatric ME reports in Korea Adverse Event Reporting System (KAERS) database from 1989 to 2012 and 208 ME reports in pediatrics were found. Based on KAERS database, the proportion of reported pediatric ME in adverse drug event (ADE) reports was 2.73 times (95% CI, 2.35-3.17) higher than that of adult ME. In 208 ME reports, we found a total of 236 ME-related terms within 19 types of MEs. The most common type of MEs was "accidental overdose" (n = 58, 24.6%), followed by "drug maladministration" (n = 50, 21.2%) and "medication error" (n = 41, 17.4%). After the narratives of ME reports were reviewed, we noticed that most of them did no harm to patients, but some cases were needed for medical treatment. Our data suggest that MEs in pediatrics are not negligible in Korea. We expect that this study would increase the awareness of the problem in pediatric MEs and induce the need for further development of an effective national ME preventing system in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Kopelman LM, Moskop JC. East Carolina University School of Medicine. Children and health care moral and social issues. Dordrecht; Boston: Kluwer Academic Publishers;1989.2. Mathis LL, Iyasu S. Safety monitoring of drugs granted exclusivity under the Best Pharmaceuticals for Children Act: what the FDA has learned. Clin Pharmacol Ther. 2007; 82:133–134.3. Kauffman RE. Clinical trials in children: problems and pitfalls. Paediatr Drugs. 2000; 2:411–418.4. Neubert A. Pharmacovigilance in pediatrics: current challenges. Paediatr Drugs. 2012; 14:1–5.5. Kohn LT, Corrigan JM, Donaldson MS. To err is human building a safer health system. Washington, D.C.: National Academy Press;2000.6. The National Coordinating Council for Medication Error Reporting and Prevention. The council: Moving into the second decade. accessed on 30 January 2015. Available at http://www.nccmerp.org/sites/default/files/fifteen_year_report.pdf.7. Safe Kids Worldwide. An in-depth look at keeping young children safe around medicine. accessed on 11 July 2013. Available at http://www.safekids.org/medsreport.8. Marino BL, Reinhardt K, Eichelberger WJ, Steingard R. Prevalence of errors in a pediatric hospital medication system: implications for error proofing. Outcomes Manag Nurs Pract. 2000; 4:129–135.9. Hicks RW, Becker SC, Cousins DD. Harmful medication errors in children: a 5-year analysis of data from the USP's MEDMARX program. J Pediatr Nurs. 2006; 21:290–298.10. Goldmann D, Kaushal R. Time to tackle the tough issues in patient safety. Pediatrics. 2002; 110:823–826.11. Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, Goldmann DA. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001; 285:2114–2120.12. WHO Collaboration Centre for Drug Statistics Methodology. Structure and Principles. accessed on 8 October 2013. Available at http://www.whocc.no/atc/structure_and_principles/.13. Cowley E, Williams R, Cousins D. Medication errors in children: a descriptive summary of medication error reports submitted to the United States pharmacopeia. Curr Ther Res. 2001; 62:627–640.14. Wong IC, Ghaleb MA, Franklin BD, Barber N. Incidence and nature of dosing errors in paediatric medications: a systematic review. Drug Saf. 2004; 27:661–670.15. Folli HL, Poole RL, Benitz WE, Russo JC. Medication error prevention by clinical pharmacists in two children's hospitals. Pediatrics. 1987; 79:718–722.16. Wilson DG, McArtney RG, Newcombe RG, McArtney RJ, Gracie J, Kirk CR, Stuart AG. Medication errors in paediatric practice: insights from a continuous quality improvement approach. Eur J Pediatr. 1998; 157:769–774.17. Wong I, Sweis D, Cope J, Florence A. Paediatric medicines research in the UK: how to move forward? Drug Saf. 2003; 26:529–537.18. Hibbard JH, Peters E, Slovic P, Tusler M. Can patients be part of the solution? Views on their role in preventing medical errors. Med Care Res Rev. 2005; 62:601–616.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Signal Detection and Safety Information Generation of Aripiprazole in Spontaneous Adverse Event Reports Database
- Patient safety management in the medication use process: prevention and management of medication error
- Analysis of Important Medical Adverse Events and Signals Related with Cyclosporine and Tacrolimus Using the FDA Adverse Event Reporting System (FAERS) Database
- Signal Detection of Adverse Event of Metoclopramide in Korea Adverse Event Reporting System (KAERS)
- Identifying the Patterns of Adverse Drug Responses of Cetuximab